Ensuring Data Security in Healthcare Platforms.

Stop Data Breaches: Why Healthcare Security is Non-Negotiable
A secure healthcare data platform is a specialized infrastructure designed to safely manage and analyze sensitive health information while meeting strict regulations like HIPAA and GDPR. These platforms provide end-to-end encryption, role-based access controls, compliance automation, audit trails, data anonymization, and support for interoperability standards like FHIR and HL7v2.
Healthcare data breaches are a constant threat. Over 14 years of PHI records have been breached, exposing millions to identity theft. For organizations handling EHR, genomics, and claims data, a single breach can trigger fines exceeding $1.35 million, destroy patient trust, and halt critical research.
But security isn’t just about avoiding penalties. Without secure infrastructure, innovation stops. Researchers can’t access the diverse datasets needed for precision medicine, AI models can’t be trained on real-world data, and patients lose control over their most sensitive information.
Global pharma, regulatory bodies, and public sector organizations face the challenge of managing siloed, inconsistent datasets. Traditional approaches require moving data, which creates significant security risks and regulatory problems.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. We built a federated secure healthcare data platform that enables in situ analytics across distributed data sources for organizations like the FDA and NHS. My 15 years in computational biology have taught me that true data security requires a complete rethinking of how we access, analyze, and govern health data at scale.
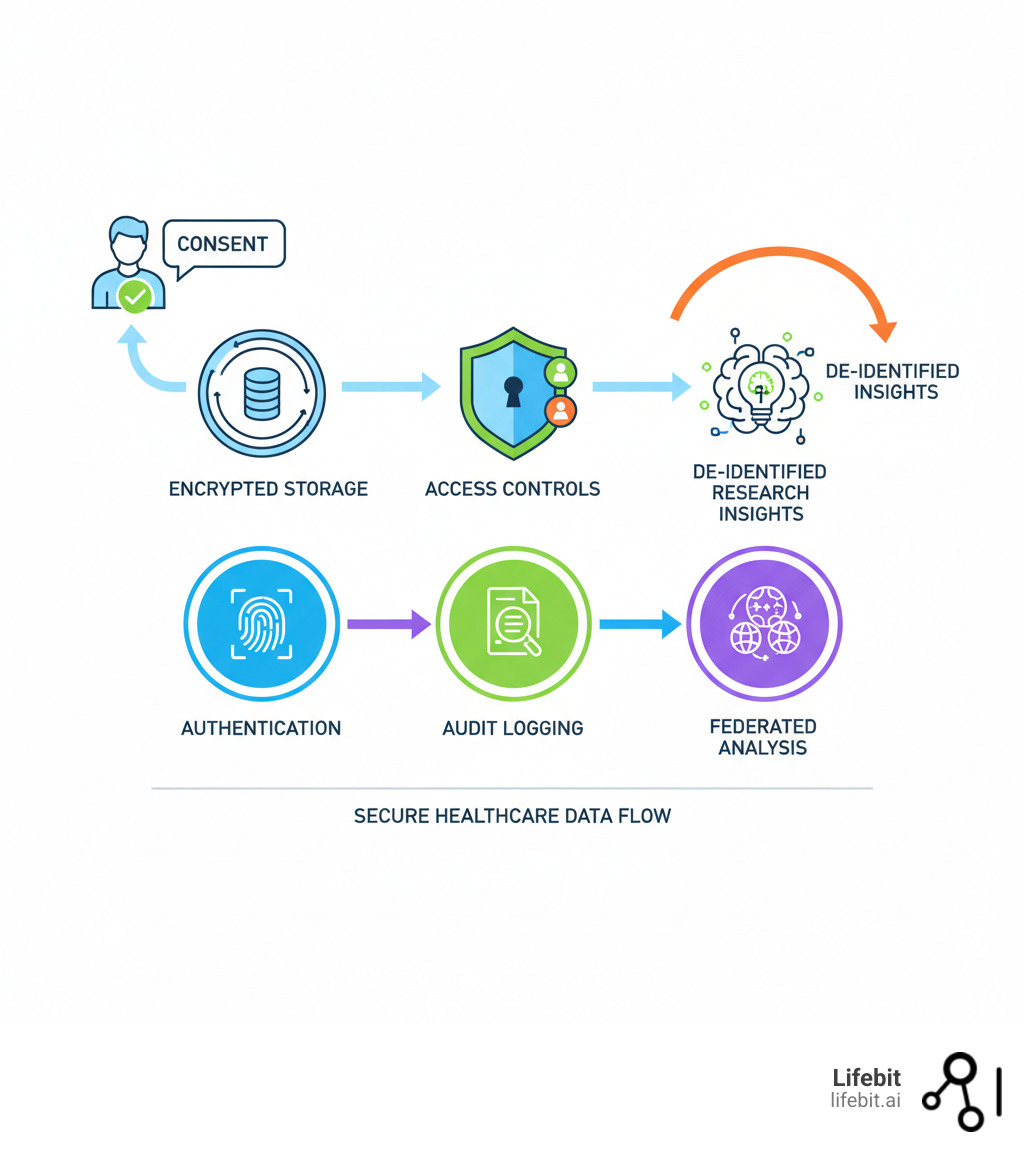
Secure healthcare data platform terms you need:
The Anatomy of a Secure Platform: 5 Core Components
A secure healthcare data platform is the central nervous system of modern healthcare, making information from countless sources accessible, analyzable, and actionable while keeping it locked down. It manages the complete data journey, which consists of five core stages. Secure ingestion involves using validated pipelines and secure APIs to bring data into the platform from disparate sources like hospital EHRs or genomic sequencers. Data change is the crucial process of cleaning, standardizing, and transforming raw data into a consistent, usable format. Intelligent storage utilizes optimized data structures to house vast and varied datasets securely and cost-effectively. Robust management encompasses the governance, access control, and auditing frameworks that control how data is used. Finally, powerful analytics provides the tools and computational environments for researchers to derive insights from the data.
This unified system allows structured EHRs, insurance claims, medical images (DICOM), genomic sequences, and even handwritten notes to work together. Our Lifebit Federated Biomedical Data Platform is built to handle this complexity, creating a single source of truth to power better decisions.

Key Functionalities and Features
What separates a basic database from an effective secure healthcare data platform?
Data harmonization and curation transforms messy, inconsistent information into structured, research-ready formats. Raw healthcare data is notoriously chaotic, with variations in diagnostic codes, measurement units (e.g., lbs vs. kg), and unstructured clinical notes. Harmonization involves mapping this data to common ontologies and terminologies like SNOMED CT for diagnoses, LOINC for lab tests, and RxNorm for medications. The platform then transforms the cleaned data into interoperable formats like FHIR resources. This intensive cleaning process is a core value of the platform, as it means scientists can start analyzing immediately instead of spending 80% of their time on data preparation.
Scalable cloud infrastructure is essential. Modern platforms use serverless architectures to handle exploding data volumes, processing billions of resources daily. This managed scalability ensures high availability and disaster recovery without manual intervention.
Data management and storage provides fully managed services for ingesting and storing various data formats (FHIR, HL7v2, DICOM). A key architectural innovation is the Trusted Data Lakehouse (TDL), which combines the scalability and flexibility of a data lake (for storing raw, multi-modal data) with the structured management features of a data warehouse. What makes it “trusted” is the integration of fine-grained security, governance, and audit capabilities directly into the storage layer. This ensures that all data, whether structured or unstructured, is subject to the same rigorous controls from the moment it is ingested. This approach, as demonstrated by the Health Data Platform British Columbia (HDPBC), provides a secure, single source of truth. Learn more in our Data Lakehouse Best Practices.
Advanced analytics tools offer customized remote desktop environments for data consumers to analyze data and manage code. Results export is a controlled process requiring approval, ensuring security is never compromised.
Platform Types for Different Needs
A secure healthcare data platform comes in several flavors:
- Research platforms provide a unified access point to diverse health datasets for public sector and academic users, like HDPBC.
- Trusted Research Environments (TREs) are highly secure, internet-free environments that let researchers analyze data without seeing raw information. All data movement is reviewed by humans. Learn more in our guide to Trusted Research Environments.
- Clinical application backends provide the secure, compliant infrastructure for patient-facing apps and EHRs.
- Analytics and AI platforms are built for machine learning, integrating with tools like BigQuery and Vertex AI to extract insights from unstructured text.
Ensuring Seamless Data Interoperability
Data interoperability—the ability of different systems to exchange and use data—is what open ups its true value.
- FHIR Resources (Fast Healthcare Interoperability Resources) are the modern standard for exchanging health information electronically, creating a unified data view.
- HL7v2 Standard is an older but still widely used messaging standard. Modern platforms support both to ensure compatibility with legacy systems.
- DICOM for imaging is the standard for handling medical images (MRIs, CT scans). Platforms integrate with imaging devices and PACS solutions to make images as accessible as other data.
Data exchange and standardization deliver real impact by linking disparate datasets like claims, EHRs, and genomics. Research shows that linking claims data to EHRs for metastatic breast cancer patients improves diagnosis coverage. Our platform facilitates this by normalizing data into a FHIR common data model, turning scattered information into a comprehensive knowledge base.
Bulletproof Your Data: Achieving Ironclad Security & Compliance
Trust is the currency of healthcare. A secure healthcare data platform builds an ecosystem where data is protected, privacy is respected, and regulations are met without compromise. Security involves the technical safeguards against breaches, while compliance is about meeting legal frameworks like HIPAA and GDPR. You need both.
At Lifebit, we integrate end-to-end encryption, zero-trust architecture, and privacy-enhancing technologies with rigorous compliance frameworks. Serving organizations like the FDA and NHS has taught us that compliance is a continuous commitment requiring automated monitoring and robust governance.
How Platforms Guarantee Data Security and Privacy
Multiple layers of protection create a fortress around patient information.
- Encryption is non-negotiable. All data is encrypted both in transit (using protocols like TLS 1.2+) and at rest (using standards like AES-256). This renders it unreadable to unauthorized parties. The best platforms are end-to-end encrypted, meaning even the platform provider cannot access the raw data. This is often achieved through robust key management systems where data owners retain control of their encryption keys, ensuring an additional layer of security and trust.
- Access control determines who sees what. Granular Role-Based Access Control (RBAC) and robust Identity and Access Management (IAM) systems are crucial. These ensure users only get the minimum permissions they absolutely need to perform their jobs (the principle of least privilege). This can be configured down to the level of individual data fields and is often integrated with institutional directories like Active Directory for streamlined user management.
- Zero-trust architecture assumes every request is a threat until verified. This model dramatically reduces the attack surface by requiring verification at every access point.
- Privacy-enhancing technologies (PETs) add layers of protection. Beyond secure enclaves (isolated processing environments) and data tokenization (which replaces sensitive information with non-sensitive substitutes), advanced platforms incorporate other PETs. Differential privacy is a mathematical technique that adds statistical noise to query results, making it impossible to re-identify any single individual while preserving the overall statistical accuracy of the analysis. Homomorphic encryption is an emerging technology that allows computations to be performed directly on encrypted data without decrypting it first, offering an unparalleled level of security for sensitive calculations. These technologies allow for powerful analysis while providing mathematical guarantees of privacy.
- Anonymization by design reduces re-identification risk while maintaining data utility. Platforms like HDPBC ensure all information undergoes human review before leaving the secure environment.
For more details, explore our approach to Secure Clinical Data.
The Critical Role of a secure healthcare data platform in Compliance
Compliance is the framework that makes innovation possible.
- HIPAA compliance is the foundation for any platform operating in the U.S. This involves adhering to several key regulations. The HIPAA Privacy Rule sets national standards for when protected health information (PHI) may be used and disclosed. The HIPAA Security Rule mandates specific technical, physical, and administrative safeguards to ensure the confidentiality, integrity, and security of electronic PHI. The Breach Notification Rule requires notification to patients and authorities following a data breach. A compliant platform automates adherence to these rules, and vendors sign Business Associate Agreements (BAAs), which are legally binding contracts that obligate them to protect PHI according to HIPAA standards.
- GDPR compliance governs data handling for individuals in the EU. Our GDPR Compliant Data practices ensure all information is processed lawfully and transparently.
- Regional and emerging regulations like California’s CCPA and the European Health Data Space Regulation (EHDS) add complexity. Our platform is EHDS-ready, anticipating future standards.
- Automated compliance monitoring provides continuous 24/7 security oversight. This isn’t a one-time check; it’s a dynamic process. The platform continuously scans its configuration against hundreds of controls mapped to standards like HIPAA, GDPR, and ISO 27001. If a misconfiguration occurs (e.g., a storage bucket is accidentally made public), the system can generate an immediate alert or even automatically remediate the issue. This proactive approach flags potential gaps before they become violations. Furthermore, comprehensive audit controls create an immutable, time-stamped record of every action—who accessed what data, when, and for what purpose—providing a complete trail for regulatory scrutiny and forensic analysis.
Leading platforms maintain certifications like HITRUST® R2, ISO 27001, and SOC 2 Type II, representing independent verification of their security and operational standards. For more, see our Healthcare Data Compliance guide.
From Data to Findy: The Real-World Impact of Secure Platforms
The real value of a secure healthcare data platform is turning data into breakthroughs. These platforms become engines of innovation, accelerating research and improving patient care.

- For researchers, platforms break down barriers to findy. Instead of wrestling with data access and formats, scientists can focus on analysis. Access to diverse, real-world data leads to accelerated findy and faster treatments.
- For healthcare organizations, operational efficiency improves, care coordination becomes easier, and regulatory risks decrease. Platforms like HDPBC provide a unified access point to diverse health datasets, streamlining complex processes.
- For patients, these platforms provide control over personal health information and pave the way for personalized medicine, where treatments are custom to an individual’s unique profile.
The Advantages of Trusted Research Environments benefit all stakeholders by fostering innovation without compromising privacy.
Powering AI and Machine Learning in Healthcare
A secure healthcare data platform provides the clean, harmonized, and diverse data that AI and machine learning models need to function.
- Training AI models on multi-modal data (clinical records, genomics, imaging) allows them to learn from the full richness of patient information. Platforms integrate with tools like BigQuery, AutoML, and Vertex AI for advanced analysis.
- Predictive analytics becomes possible by analyzing longitudinal data, allowing algorithms to spot patterns humans might miss. For example, models can be trained to predict sepsis risk in ICU patients by analyzing real-time streams of vital signs and lab results, enabling earlier intervention. Other models can identify patients at high risk for hospital readmission, allowing care teams to provide targeted post-discharge support. In public health, predictive analytics can forecast infectious disease outbreaks by analyzing anonymized EHR and mobility data, giving authorities a critical head start.
- Drug findy is being transformed by real-world data. Evidence on how medications perform outside of clinical trials, like apalutamide’s effect on prostate cancer, is revolutionizing drug development and pharmacovigilance.
- Federated data analysis is a cutting-edge, privacy-preserving technique that is central to modern collaborative research. It allows models to train on distributed datasets without the raw data ever leaving its source institution’s secure perimeter. The process works by sending the analytical model or query to each data location. The model trains locally on the data, and only the resulting aggregated parameters or model updates—which do not contain any individual-level information—are sent back to a central server to be combined. This allows researchers to build a powerful, global model from diverse datasets while ensuring that sensitive patient data remains fully under the control of the local data custodian. Our Federated Data Analysis approach enables this powerful form of collaboration while maintaining maximum data control and security.
For example, research using CGM data from EHRs for diabetes studies identified previously invisible hypoglycemic events, leading to new treatment strategies. This is AI in action.
What is the role of a secure healthcare data platform in research?
A secure healthcare data platform is essential for modern research.
- Secure Data Environments (SDEs), or Trusted Research Environments (TREs), create protected spaces where researchers can work with sensitive data without directly accessing it. This balances access with protection, as detailed in our guide to creating a Secure Research Environment.
- Data linkage connects a patient’s disparate records—such as their EHR, insurance claims, lab results, and genomic data—to create a comprehensive, longitudinal view of their health journey. This is a significant technical challenge, as there is often no single universal patient identifier across different systems. Secure platforms address this using techniques like Privacy-Preserving Record Linkage (PPRL). This involves creating encrypted or tokenized identifiers from patient-identifying information. These tokens can be matched across datasets to link records without ever exposing the raw personal data, enabling powerful research into how diseases progress and which treatments work best over time.
- Collaborative research across institutions becomes practical, with built-in governance frameworks ensuring everyone follows the same rules.
- Access to real-world data shows what happens when actual patients receive treatments, providing critical evidence for regulatory decisions and clinical guidelines.
- Reproducible research is ensured through controlled environments with versioned tools and complete audit trails, which is fundamental to scientific progress.
Your Implementation Roadmap: Avoid These Costly Mistakes
Adopting a secure healthcare data platform is a strategic change, not a simple software installation. Most organizations deal with a patchwork of legacy systems, making unification a complex but valuable endeavor.
Implementation Challenges
- Data migration and legacy system integration: Harmonizing data from different formats and connecting systems that weren’t designed to communicate requires deep expertise and patience.
- User adoption: Without proper training and a clear value proposition, clinicians and researchers may stick to old workflows, leaving the new platform underused.
- Establishing robust governance: Defining who accesses what data, for how long, and under what approvals is an organizational challenge that requires buy-in from all stakeholders. Our Data Governance Platform Complete Guide can help.
Key Considerations
Before committing to a platform, consider these critical factors:
- Scalability: Your platform must grow with your data. Look for solutions with managed scalability and serverless architecture that can handle increasing volumes without performance degradation.
- Total cost of ownership (TCO): Look beyond the sticker price to include maintenance, support, training, and integration costs. Cloud solutions often offer predictable pay-as-you-go models.
- Vendor expertise and support: You need a partner who understands the nuances of healthcare data, regulations like HIPAA, and the complexities of real-world health data. Look for vendors with comprehensive support and professional services.
- Data residency: Some regulations require patient data to remain within national borders. Modern platforms offer flexible cloud, on-premises, or hybrid deployments to control where your data physically resides.
| Deployment Model | Cost | Scalability | Control |
|---|---|---|---|
| Cloud-Based | Medium | High | Medium |
| On-Premise | High | Low | High |
| Hybrid | Medium | Medium | Medium |
The hybrid approach is increasingly popular, balancing the security of on-premises storage with the scalability of cloud analytics.
The Future is Federated: What’s Next for Your Data Strategy?
The next generation of secure healthcare data platform architecture is federated. Instead of copying sensitive data to central repositories—creating security risks—federated systems bring the analysis to where the data lives.

This is federated learning in action. Machine learning algorithms travel to each data source, learn locally, and share only insights—never the raw data. This allows for analysis across millions of patient records without data ever leaving its secure home. Our R.E.A.L. (Real-time Evidence & Analytics Layer) already enables AI-driven safety surveillance across distributed systems this way.
The shift toward real-time analytics is also transformative. Modern platforms process data as it arrives, enabling immediate insights for pharmacovigilance and public health surveillance. Potential safety signals can be investigated in hours, not months.
Patients are becoming more central to this evolution. Future platforms will give individuals meaningful control over their health data, balancing privacy rights with the collective benefit of advancing science.
Regulatory frameworks like the European Health Data Space Regulation (EHDS) are evolving to support this vision, creating a unified market for health data that will accelerate cross-border collaboration.
Trusted Research Environments will become the default. As explored in What is a Secure Data Environment (SDE)?, these controlled environments ensure sensitive analysis happens within governed boundaries.
The impact on innovation will be profound. Precision medicine will scale, public health agencies will detect outbreaks faster, and health crises will be met with unprecedented speed. We are building interconnected data ecosystems where data stays local, governance stays strong, and insights flow freely. This is how we open up the full potential of real-world data—by reimagining what a secure healthcare data platform can be.
Frequently Asked Questions about Secure Healthcare Data Platforms
Here are answers to the most common questions we hear from clinics, research labs, and healthcare organizations.
What’s the difference between a regular cloud database and a secure healthcare data platform?
A regular cloud database is a generic storage container. A secure healthcare data platform is purpose-built for health information with key differences:
- Compliance-First Design: Built from the ground up to meet HIPAA, GDPR, and other regulations.
- Built-in Security: Includes end-to-end encryption, role-based access control (RBAC), and advanced identity management as standard features.
- Healthcare-Native: Offers native support for standards like FHIR, HL7v2, and DICOM for seamless interoperability.
- Complete Audit Trails: Tracks every data access and modification with immutable logs for regulatory accountability.
In short, a generic database stores data. A secure healthcare platform protects, governs, and makes it research-ready while ensuring compliance.
How do these platforms handle patient consent?
Modern platforms build consent management directly into their architecture. They include dedicated consent management modules that digitally capture, store, and track patient preferences. The best platforms support granular permissions, allowing patients to specify exactly how their data can be used (e.g., for clinical care vs. specific research studies). Adherence to regulations like GDPR is built-in, and some platforms offer patient-facing portals for individuals to manage their consent, enhancing transparency and trust.
Can small clinics or research labs afford these platforms?
Yes. The shift to cloud-based models has made enterprise-grade security and compliance accessible to organizations of all sizes.
- Scalable Pricing: Many platforms operate on pay-as-you-go models, so you only pay for the storage and compute resources you use. This keeps costs aligned with actual usage.
- No Upfront Investment: Cloud-based SaaS options eliminate the need for massive investments in servers and other hardware.
- Reduced Overhead: The vendor manages the infrastructure, security updates, and compliance monitoring, allowing your team to focus on research and patient care instead of IT.
The question isn’t whether small organizations can afford these platforms, but whether they can afford the security and compliance risks of not having one.
Your Next Step: Secure Your Data, Accelerate Your Research
Healthcare data holds the promise to cure diseases and save lives, but mishandling it can destroy patient trust and halt innovation. A secure healthcare data platform is the essential foundation for the future of medicine.
These platforms transform siloed data into actionable insights through end-to-end encryption, robust access controls, and compliance automation. By enabling advanced analytics and federated learning, they allow for groundbreaking collaboration without compromising privacy.
When you get security right, everything else becomes possible. Federated data ecosystems are emerging, real-time analytics are catching adverse events before they become crises, and patient-centric models are putting individuals in control.
Choosing the right partner is key. You need a platform that offers managed scalability, comprehensive compliance, and deep expertise in the complexities of healthcare data.
At Lifebit, we built our federated AI platform for this challenge. Our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) provide secure, real-time access to global biomedical data. We power large-scale, compliant research and pharmacovigilance for biopharma, governments, and public health agencies worldwide. The future of healthcare depends on our ability to open up insights while fiercely protecting privacy. We’re here to help you build that future.

