Stop Missing the Signs with Modern Signal Detection Software

Why Missing Safety Signals Puts Patients and Billions at Risk
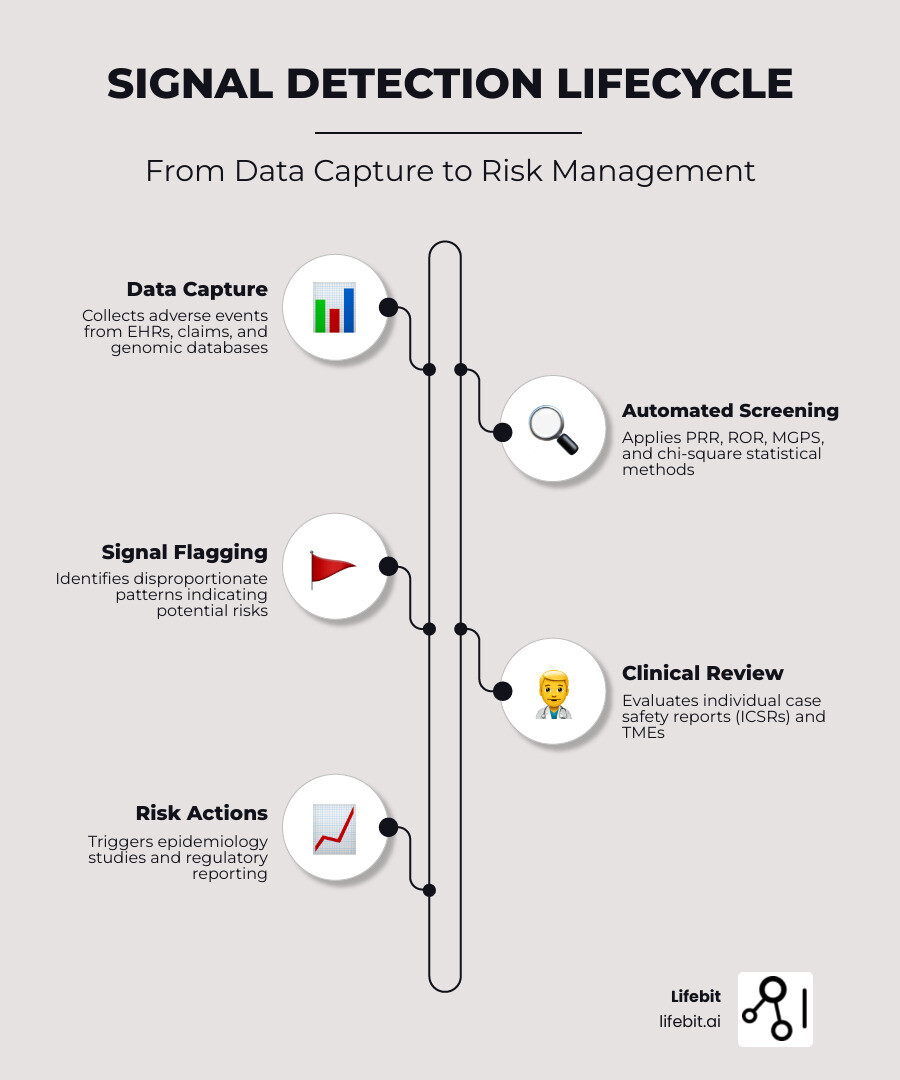
Signal detection software is a specialized platform that continuously monitors health data—including adverse event reports, electronic health records, claims, and genomic datasets—to identify unexpected patterns that may indicate drug safety risks, disease outbreaks, or treatment failures. These tools combine statistical methods like disproportionality analysis (PRR, ROR, MGPS) with machine learning to flag potential safety concerns before they escalate into public health crises or regulatory action.
What signal detection software does:
- Automates real-time analysis of multiple diverse data sources to detect new or changing safety risks
- Performs quantitative screening using statistical methods (PRR, ROR, MGPS, chi-square) to identify signals faster than manual review
- Integrates qualitative clinical evaluation of individual case safety reports (ICSRs) and targeted medical events (TMEs)
- Supports regulatory compliance with GVP modules, 21 CFR Part 11, and automated audit trails
- Scales from small research teams to enterprise deployments handling 500,000+ users and 120,000+ cases
- Enables federated analysis across siloed datasets without moving sensitive patient data
The stakes are high. Traditional manual safety reviews are slow, inconsistent, and prone to missing rare but serious adverse events buried in massive datasets. A single missed signal can lead to preventable patient harm, regulatory penalties, and billions in liability costs. Meanwhile, pharmaceutical companies and public health agencies struggle with fragmented data across EHRs, claims systems, and genomic databases—making it nearly impossible to connect the dots in real time.
Modern signal detection software addresses these challenges by automating the heavy lifting of safety surveillance while preserving the clinical judgment that separates statistical noise from true safety concerns. Advanced enterprise platforms now serve hundreds of thousands of users globally, delivering interactive visualizations, collaborative workspaces, and end-to-end signal management in a single environment.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built a federated AI platform that enables real-time signal detection software across secure, compliant environments for global pharma and public health leaders. Over 15 years working in computational biology, genomics, and health-tech, I’ve seen how the right infrastructure transforms safety surveillance from reactive guesswork into proactive, evidence-driven protection.
Know your Signal detection software terms:
What is Signal Detection Software and Why Does It Matter?
At its core, Signal detection software is the “early warning system” for the life sciences industry. In pharmacovigilance, a “signal” is information that suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event. Whether we are talking about a pharmaceutical giant monitoring a newly launched blockbuster drug or a public health agency tracking a viral outbreak, the goal is the same: find the needle in the haystack before the haystack catches fire.
The Evolution of Pharmacovigilance
Historically, drug safety relied on spontaneous reporting systems where healthcare providers or patients would manually submit reports of adverse events. This reactive model was limited by under-reporting, poor data quality, and significant time lags. The introduction of signal detection software has shifted the paradigm toward a proactive, data-driven approach. Modern tools enable proactive risk screening by scanning numerous health outcomes that occur after exposure to a medical product. This is essential for post-marketing drug surveillance where the population using a drug is much larger and more diverse than the group studied in clinical trials.
In the past, signal detection was a labor-intensive process of manual clinical review. Today, the sheer volume of data—from millions of Individual Case Safety Reports (ICSRs) to petabytes of multi-omic data—makes manual review impossible. For more on how this fits into a broader strategy, see our AI for pharmacovigilance complete guide.
How Signal Detection Software Transforms Modern Safety
The transition from reactive to proactive safety is powered by several key technological shifts:
- Automated Analytics: Software can now process millions of records in seconds, identifying statistical outliers that human eyes would miss. This includes the use of disproportionality analysis to flag events that occur more frequently than expected.
- Multi-source Data Integration: Modern platforms don’t just look at spontaneous reports; they integrate data from literature, social media, EHRs, and claims. This “Real-World Data” (RWD) provides a more comprehensive view of patient health outside of controlled environments.
- End-to-End Signal Management: Leading tools provide a single collaborative workspace where a signal can be detected, documented, evaluated, and tracked through its entire lifecycle. This ensures that no potential risk is lost in administrative silos.
- Epidemiology Support: Once a signal is identified, the software often provides the groundwork for follow-up epidemiology safety studies to confirm the risk. This might involve cohort studies or case-control analyses conducted within the same software environment.
- Predictive Modeling: Advanced platforms are beginning to use historical data to predict which drugs or patient populations are at the highest risk for specific adverse events, allowing for targeted monitoring strategies.
Quantitative vs. Qualitative Methods in Signal Detection Software
Effective Signal detection software must balance two distinct but complementary approaches: quantitative data mining and qualitative clinical review. The synergy between these two methods allows safety teams to filter out statistical noise while ensuring that rare, clinically significant events are not overlooked.
Advanced Statistical Algorithms
Quantitative methods rely on “disproportionality analysis.” This involves looking for combinations of drugs and adverse events that occur more frequently in a database than would be expected by chance. Common algorithms include:
- PRR (Proportional Reporting Ratio): A simple ratio comparing the frequency of a specific event for a drug against the frequency of that event for all other drugs. It is widely used for its simplicity and ease of interpretation.
- ROR (Reporting Odds Ratio): Similar to an odds ratio in epidemiology, it provides a measure of the strength of the association between the drug and the event.
- MGPS (Multi-item Gamma Poisson Shrinker): A sophisticated Bayesian method used by regulatory bodies like the FDA. MGPS is particularly effective at reducing “noise” from small sample sizes by applying a shrinkage factor to the estimates, which prevents rare events from producing artificially high scores.
- BCPNN (Bayesian Confidence Propagation Neural Network): Used by the Uppsala Monitoring Centre (UMC), this method calculates the Information Component (IC), which measures the difference between the observed and expected reporting rates.
Qualitative Clinical Evaluation
Qualitative methods focus on the clinical nuances. This includes the review of individual cases (ICSRs) to look for a “strong” clinical story. Key factors in qualitative review include:
- Temporal Relationship: Does the event occur within a biologically plausible timeframe after drug administration?
- De-challenge and Re-challenge: Does the event stop when the drug is discontinued, and does it return if the drug is restarted? These are powerful indicators of causality.
- Medical History: Are there confounding factors, such as pre-existing conditions or concomitant medications, that could explain the event?
| Feature | Quantitative Data Mining | Qualitative Clinical Evaluation |
|---|---|---|
| Primary Goal | Identify statistical outliers/trends | Determine clinical causality |
| Data Source | Aggregated databases (e.g., FAERS, VigiBase) | Individual Case Safety Reports (ICSRs) |
| Strengths | Fast, handles massive datasets, objective | Context-aware, identifies rare “smoking guns” |
| Weaknesses | Can produce false positives (noise) | Slow, subjective, not scalable manually |
Signal Prioritization Frameworks
Not every statistical flag is a true safety signal. Signal detection software helps teams prioritize findings using frameworks like the Signal Prioritization Matrix. This involves assessing the “Impact” (severity of the event, public health implications) against the “Strength of Evidence” (statistical significance, clinical plausibility). High-impact, high-evidence signals are fast-tracked for immediate regulatory action, while low-impact signals may be placed on a “watch list” for continued monitoring.
Core Capabilities of Lifebit Signal Detection Software
At Lifebit, we believe that Signal detection software shouldn’t just be about finding risks—it should be about managing them securely and efficiently. Our pharmacovigilance platform is designed to handle the complexity of modern biomedical data without compromising on security.
The Signal Management Lifecycle
Our software supports the entire signal management lifecycle as defined by CIOMS and GVP Module IX:
- Signal Detection: Automated scanning of diverse datasets using advanced disproportionality algorithms.
- Signal Validation: Tools for medical reviewers to quickly assess whether a statistical flag represents a new potential risk or a known side effect.
- Signal Analysis and Prioritization: Categorizing signals based on their potential impact on patient safety and the strength of the available evidence.
- Signal Assessment: Integrating clinical trial data, literature, and real-world evidence to determine if a causal relationship is likely.
- Recommendation for Action: Generating automated reports for regulatory submissions or internal risk management planning.
Regulatory Compliance and GVP Modules
One of the standout features of our approach is the integration of GVP (Good Pharmacovigilance Practices) modules. These ensure that every step of the signal management process is conducted according to global regulatory standards. Furthermore, our platform is fully 21 CFR Part 11 compliant, providing the necessary electronic signatures and audit trails required for regulatory submissions. This compliance is critical for maintaining the integrity of safety data and ensuring that all decisions are traceable and defensible during audits.
For a deeper dive into compliance, check out our pharmacovigilance compliance solution.
Enterprise-Ready Features for Drug Safety
To support large-scale operations, our software includes:
- Targeted Medical Events (TMEs) & AESIs: Automated flagging of Adverse Events of Special Interest (AESIs) during the initial assessment phase, ensuring that critical events like anaphylaxis or liver injury are never missed.
- Workflow Integration: Configurable workflows that align with your team’s specific risk management processes, allowing for seamless collaboration between safety scientists and medical reviewers.
- Scalability: Our federated architecture allows us to support hundreds of thousands of users and millions of cases across five continents without the need to move sensitive patient data.
- Automated Notifications: Real-time alerts when a pre-defined statistical threshold (like a specific PRR score or a change in the IC value) is reached.
Specialized Signal Detection for Genomics and Medical Data
The term “signal detection” isn’t limited to adverse drug reactions. In genomics and medical research, detecting signals means identifying meaningful biological patterns within massive datasets.
In fields like oncology and rare disease research, Signal detection software is used for real-time anomaly detection in genomic sequences. This requires massive bandwidth—often processing up to 500MHz of instantaneous data in RF contexts or millions of genomic “reads” in sequencing. By identifying these signals rapidly, researchers can pinpoint disease markers or potential drug targets with unprecedented speed.
Learn more about these applications in our AI for pharmacovigilance complete guide.
Advanced Genomic and Medical Data Analysis
For researchers working with ChIP-Seq and ATAC-Seq data, signal detection takes the form of motif detection. This involves:
- Known Motif Searching: Using databases like JASPAR to find enrichment of known transcription factor binding sites.
- De Novo Motif Detection: Using algorithms like Gibbs sampling to find entirely new patterns. This stochastic procedure iteratively maximizes the log-likelihood ratio (LLR) to find the most significant motifs within a set of genomic regions.
- Quality Scoring: Normalizing scores to ensure that finded motifs are biologically relevant and not just random sequence repetitions.
Technical Requirements and Integration Standards
Running a high-performance Signal detection software suite requires more than just a standard laptop. To handle real-time spectrum analysis or large-scale pharmacovigilance databases, specific hardware and software standards must be met to ensure data integrity and system responsiveness.
Hardware and Performance Specifications
High-performance signal analysis tools require Intel i7 processors (or equivalent) and OpenGL 3.0 support to maintain real-time interactive visualizations. In the Linux environment, users often need to manually increase USB memory allocation (sometimes up to 32MB or more) to prevent data loss when running multiple high-speed devices on a single bus. For enterprise deployments, high-speed SSDs and significant RAM (64GB+) are recommended to handle the indexing of millions of safety reports.
Data Standards and Interoperability
Integration is equally critical. Modern platforms must support a variety of data standards to ensure they can communicate with other systems in the healthcare ecosystem:
- REST APIs: For connecting to external data sources, such as the FDA’s openFDA portal or third-party literature databases.
- HL7 FHIR: The standard for exchanging electronic health records, allowing signal detection software to pull real-world evidence directly from hospital systems.
- OMOP Common Data Model (CDM): Essential for federated analysis. By standardizing data into the OMOP format, researchers can run the same signal detection algorithms across different databases globally without moving the data.
- JSON & VITA-49: Standards that ensure data interoperability across different hardware and software systems, particularly in RF and high-speed data streaming contexts.
- Missing Value Standardization: Critical for clinical databases where consistent coding (e.g., using -9999 for missing values) prevents errors in ROC analysis and ensures that statistical models are trained on clean data.
Deployment and Infrastructure
We offer flexible deployment to meet different security and infrastructure needs:
- Cloud-native: Leveraging AWS, Azure, or GCP for maximum scalability and ease of access across global teams.
- On-premises/Hybrid: For organizations with strict data residency requirements or those operating in highly regulated jurisdictions.
- Containerized: Using Docker or Kubernetes to ensure the software runs consistently across different computing environments, from a developer’s laptop to a massive server cluster.
Overcoming Challenges: Noise, Scale, and the Future of AI
The biggest enemy of Signal detection software is “noise.” In pharmacovigilance, noise can come from various biases that distort statistical results. Addressing these challenges requires a combination of advanced mathematics and domain expertise.
Addressing Bias and Confounding Factors
- Notoriety Bias: This occurs when a drug gets reported more frequently because it is currently in the news or subject to a safety alert, creating a feedback loop of false signals.
- The Weber Effect: A phenomenon where reporting rates for a new drug peak in the first two years after launch and then decline, regardless of the actual safety profile.
- Co-reporting: When multiple drugs are reported for the same event, it can be difficult to determine which drug (if any) is the cause. Signal detection software uses multivariate analysis to tease apart these associations.
- Protopathic Bias: Occurs when a drug is prescribed to treat the early symptoms of a disease that has not yet been diagnosed, making it appear as though the drug caused the disease.
The Role of AI and Deep Learning
To address these biases, the industry is moving toward AI/ML integration. Traditional statistical methods are being augmented by neural networks that can identify complex, non-linear patterns. For example, some RF detection tools now use AI to identify signals 1,000x faster than traditional approaches. In the biomedical space, Large Language Models (LLMs) are being used to perform “automated narrative review,” extracting clinical insights from the free-text sections of safety reports that were previously inaccessible to quantitative algorithms.
Interoperability and the Future
We are seeing the rise of ONNX (Open Neural Network Exchange) models, which allow researchers to train a model in one framework (like PyTorch) and deploy it in another. This interoperability is key to building AI-driven pharmacovigilance solutions that can scale globally. The future of signal detection lies in “Continuous Learning” systems that update their risk models in real-time as new data flows in, moving us closer to a world of truly personalized drug safety monitoring.
Frequently Asked Questions about Signal Detection Software
How do open-source tools compare to enterprise solutions?
Open-source tools and basic statistical software are excellent for academic research and small-scale pilot studies. They offer transparency and are often free to use. However, they typically lack the scalability, 24/7 technical support, and rigorous regulatory compliance (like 21 CFR Part 11) required by major pharmaceutical companies and government agencies. Enterprise solutions provide the “peace of mind” of validated workflows and end-to-end security.
What are the main use cases in clinical research?
The most common use cases include ROC (Receiver Operator Characteristic) analysis to predict binary outcomes (e.g., will this patient respond to a drug based on their genetics?). It is also used extensively for ECG/EEG analysis, where software must filter out muscle noise to find the true biological signal. Best practices in research often involve “sample splitting”—using one half of the data for exploratory signal detection and the other half for confirmatory analysis.
How does real-time signal detection work in medical data?
Real-time detection involves processing “streams” of data as they arrive. Rather than waiting for a monthly report, the software uses machine learning to compare incoming data against a baseline in real-time. If a sudden spike in a specific adverse event is detected, the system triggers an automated alert, allowing safety teams to react instantly.
Conclusion
The era of manual, reactive safety monitoring is over. Whether you are protecting patients from adverse drug reactions or hunting for the next breakthrough in genomic medicine, Signal detection software is the essential tool for the job.
At Lifebit, we are proud to power this revolution with our federated AI platform. By providing a Trusted Research Environment (TRE) and our R.E.A.L. (Real-time Evidence & Analytics Layer), we enable organizations to access global data and generate insights without ever moving sensitive information. This is how we stop missing the signs and start building a safer, healthier future.
Ready to see it in action? Explore our real-time adverse drug reaction surveillance capabilities today.

