Essential Tech Partners for Large-Scale Health Cohorts

Why Large Cohort Studies Need Better Technology Now
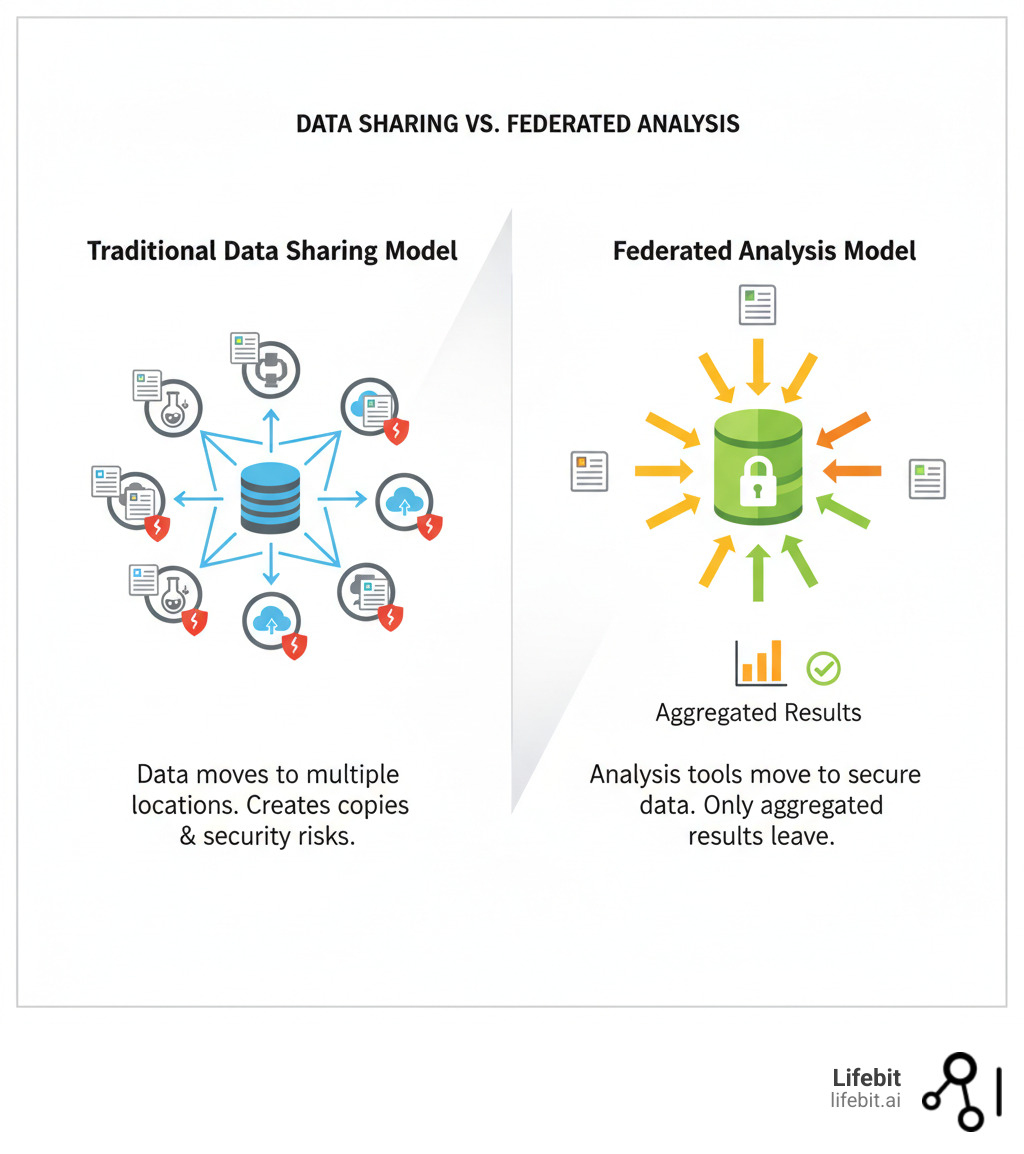
Large-scale population health studies like CanPath, with over 330,000 participants, generate enormous volumes of sensitive data. Traditional methods of moving this data create severe security risks, compliance nightmares, and research delays that can last for years. The solution is to bring analysis to the data, a model powered by key technology partners for large cohort studies like canpath.
Key Technology Partners for Large Cohort Studies:
- Federated Trusted Research Environments (TREs): Enable secure analysis where data resides, without moving sensitive information.
- Cloud Infrastructure Providers (e.g., AWS): Deliver scalable, cost-effective computing power.
- AI/ML Analytics Platforms: Power pattern recognition and predictive modeling from vast datasets.
- Data Harmonization Solutions: Standardize diverse data types across cohorts.
- Secure Collaboration Platforms: Connect distributed research teams with strict access controls.
The challenge is clear: studies like the Ontario Health Study (225,620 participants) cannot simply email sensitive files. Moving data violates privacy laws, erodes trust, and requires massive computational resources. The only viable solution is to bring the analysis to the data.
I’m Maria Chatzou Dunford, CEO of Lifebit. For over 15 years, we’ve built federated data platforms for leading institutions like Genomics England, Singapore’s Synapxe, and CanPath. My experience shows that the right infrastructure doesn’t just solve technical problems—it fundamentally accelerates scientific findy.
The Data Dilemma: Why Traditional Methods Cripple Large Cohort Studies
Managing a study like CanPath—Canada’s largest population health initiative with 330,000+ volunteers—is about stewarding personal health stories. The Ontario Health Study (OHS) alone contributes data from 225,620 participants, including questionnaires, biosamples, and linked health records. This scientific treasure trove presents immense challenges. Traditional methods of copying and moving data are crippled by staggering data volume, extreme data sensitivity, and complex regulatory compliance.
The Challenge of Managing Massive, Sensitive Datasets
The sheer scale of the data is the first hurdle. A single whole-genome sequence (WGS) can be over 200 gigabytes. For a cohort like CanPath, this translates to over 66 petabytes of raw genomic data alone. This doesn’t even account for other rich multi-omics data types like transcriptomics (gene expression), proteomics (proteins), and metabolomics (metabolites), each adding terabytes of information. Add to this high-resolution imaging data (MRIs, PET scans, digital pathology slides), continuous streams of data from wearable devices, and decades of linked electronic health records (EHRs), and the total volume becomes almost impossible to manage with traditional infrastructure.
Traditionally, every collaborating institution would need to house a complete, expensive copy. But the data sensitivity makes this approach not just impractical, but dangerous. These datasets contain identifiable genomic sequences, intimate medical histories, and personal lifestyle information. Every data transfer creates another potential point of failure, a new copy that must be secured and tracked, dramatically increasing security risks and the potential for a catastrophic data breach that would shatter participant trust.
The complexity multiplies with heterogeneous data types. Researchers must integrate structured clinical records, unstructured physician’s notes, DICOM-formatted imaging data, VCF files for genomic variants, and survey data in various formats. Data harmonization—the process of transforming this disparate information into a common format (like the OMOP Common Data Model) so it can be analyzed together—is a monumental technical puzzle. Without the right tools, this process can take months or even years, consuming valuable research time and resources before a single hypothesis can be tested.
Navigating a Maze of Regulations and Silos
Even if the technical challenges of volume and variety are solved, a legal and ethical minefield remains. International collaborations require navigating a patchwork of stringent privacy laws, including the GDPR in Europe and HIPAA in the United States. Within Canada, the situation is equally complex. Data governance is a provincial matter, meaning researchers must contend with a maze of regulations like Ontario’s PHIPA, Quebec’s Bill 64, and British Columbia’s FIPPA. Each law has different rules for data use, consent, and cross-border transfer.
This regulatory complexity, coupled with institutional policies, creates formidable data silos. Each of CanPath’s seven regional cohorts may operate under different governance structures and data access policies dictated by their host institutions and provincial laws. Gaining approval for a multi-cohort study traditionally requires separate applications to multiple Research Ethics Boards (REBs), a process notorious for its length and inconsistency. These institutional barriers and a lack of interoperability between legacy systems lead to significant data harmonization difficulties and paralyzing collaboration barriers.
The consequences are severe: research delays stretch projects from months into years. High computational costs pile up as individual institutions maintain duplicative, underutilized high-performance computing infrastructures. Most critically, when data is constantly being copied and moved, participant trust erosion becomes an existential risk. That’s why technology partners for large cohort studies like CanPath are rethinking the model entirely, asking: what if the data never had to move at all?
The Federated Revolution: A New Paradigm for Secure Data Access
The days of wrestling with massive data transfers and navigating regulatory labyrinths are over. A smarter, federated approach has emerged, changing how technology partners for large cohort studies like CanPath operate. Instead of dragging sensitive data across networks, federated systems bring the analysis tools to where the data already lives, solving long-standing problems of security, compliance, and collaboration.

How ‘Bringing the Analysis to the Data’ Works
Instead of moving evidence from a crime scene, a detective brings their tools to analyze it on-site. The federated model applies this same logic to data. At its core are Federated Trusted Research Environments (TREs), which provide approved researchers with secure workspaces—isolated, tightly controlled digital clean rooms. Within this environment, a researcher can access the data and analysis tools they have been approved to use, but they cannot download or move the raw data itself.
The process is powered by containerized analysis tools. Technologies like Docker and Singularity package applications (like scripts written in R or Python) and all their dependencies into a single, portable container. A researcher develops their analysis script locally on dummy data, packages it in a container, and submits it to the federated platform. The platform then sends this secure, self-contained analysis package to each of the distributed datasets it has been approved to run on—for example, to the Ontario cohort’s server, the BC cohort’s server, and a collaborator’s server in the UK. The analysis executes right alongside the data inside each location’s secure environment. This ensures perfect reproducibility and eliminates the headaches of software versions and dependencies.
When querying distributed data, the analysis runs in parallel in situ at each location. Only aggregated results—such as a p-value from a statistical test, a regression coefficient, or a trained machine learning model’s parameters, with no individual participant information—are returned to the researcher. To further protect privacy, advanced Privacy-Enhancing Technologies (PETs) can be employed. For instance, differential privacy adds mathematically calibrated statistical noise to the results, making it impossible to reverse-engineer the contribution of any single individual, providing a formal privacy guarantee. The raw data never moves, eliminating security vulnerabilities from data copies. Detailed audit trails track every action, ensuring transparency and maintaining participant trust. This data immobility approach delivers improved security by design.
Enabling Secure International and Cross-Country Collaboration
The federated model liberates research by enabling powerful analysis across globally distributed datasets. This is critical for statistical power, especially when studying rare diseases or diverse populations. Combining insights from hundreds of thousands of participants across different ancestries and environments helps find genetic and environmental risk factors that smaller, isolated studies would miss. The Ontario Health Study has already demonstrated this power through meta-analysis with international biobanks, but federated platforms make this process vastly more efficient and secure.
Global research networks thrive in these environments. Organizations like the Global Alliance for Genomics and Health (GA4GH) develop standards, such as the Beacon API (for data discovery) and Passports (for authentication), that create a common language for federated systems to communicate. This allows a researcher to discover relevant datasets across a global network and then run an analysis across them seamlessly. This model allows collaboration while respecting data sovereignty—the non-negotiable principle that data remains under the legal and governance jurisdiction where it was collected. For example, Genomics England’s 100,000 Genomes Project, which operates within a secure TRE, has enabled global collaboration that has led to new diagnoses for thousands of patients with rare diseases. Similarly, the All of Us Research Program in the US is building a massive cohort of over one million people, and its ‘data passport’ model for researcher access operates on similar principles of secure, controlled analysis.
Federated TREs handle this global compliance complexity by design. Analysis of UK data happens within a GDPR-compliant environment, while analysis of Canadian data adheres to provincial privacy laws. The standardized environments provided by technology partners for large cohort studies like CanPath also eliminate technical barriers, ensuring all collaborators work in consistent, validated, and reproducible systems. This is how modern population health research becomes secure, collaborative, and fast.
Essential Technology Partners for Large Cohort Studies Like CanPath
The success of large cohort studies depends on the right technological foundations and the right technology partners for large cohort studies like canpath to turn data into discovery.

Secure & Scalable Platforms: The Role of Federated TREs
Federated Trusted Research Environments (TREs) are the cornerstone of modern cohort research. They solve the central dilemma: giving researchers powerful tools while keeping participant data completely safe and stationary. A TRE is a secure digital laboratory where researchers can analyze sensitive data but can never take the raw data with them.
A best-in-class data platform for cohort studies must provide:
- Granular, role-based access controls to ensure researchers can only see and analyze the specific datasets and variables for which they have explicit ethical and governance approval.
- Customizable and reproducible analysis environments supporting a wide array of tools, from command-line interfaces for bioinformaticians to Jupyter notebooks for data scientists and graphical interfaces for clinicians.
- Secure integration for custom tools and AI models, allowing research groups to bring their own proprietary algorithms into the environment without compromising security.
- An intuitive, user-friendly interface that democratizes data access for researchers of all technical skill levels, not just computational experts.
- Robust data harmonization capabilities and tools to help standardize diverse information into common data models, drastically reducing data preparation time.
- Complete, immutable audit trails that log every action taken by every user, providing full transparency for governance bodies and participants.
- Enterprise-grade security and privacy features, including end-to-end encryption, automated de-identification, and compliance with international standards like ISO 27001.
Our federated TRE technology delivers this security and functionality, and it already powers research at leading global institutions like Genomics England, Synapxe (Singapore’s national HealthTech agency), and CanPath itself. Approved researchers can analyze CanPath data without needing massive local computing resources—the platform handles the heavy lifting in the cloud.
Powering Analysis: Cloud Infrastructure and Advanced Analytics
Cloud infrastructure from providers like Amazon Web Services (AWS), Microsoft Azure, and Google Cloud Platform (GCP) has fundamentally changed what’s possible in cohort research. They offer virtually unlimited, on-demand computing power and storage. When a researcher needs to run a complex genomic analysis on 10,000 samples, the platform can instantly provision thousands of CPU cores via services like AWS EC2 or Azure VMs, use scalable storage like AWS S3, and then scale everything back down the moment the job is finished. This pay-as-you-go cost-efficiency eliminates the need for massive upfront capital investment in on-premise servers. This reduced computational burden allows institutions to redirect budgets from maintaining server rooms to funding more science.
But raw computing power isn’t enough. You need AI and machine learning to find the needles in the petabyte-scale data haystack. Our platform integrates these AI/ML capabilities directly into the TRE, allowing researchers to deploy sophisticated algorithms while maintaining the highest security standards.
- Powering Large-Scale GWAS: Federated platforms enable Genome-Wide Association Studies (GWAS) to be run across multiple international cohorts simultaneously. This massive increase in sample size provides the statistical power needed to identify genetic variants associated with disease, especially rare variants with small effect sizes.
- Developing Equitable Polygenic Risk Scores (PRS): PRS, which aggregate the effects of many genetic variants to predict an individual’s risk for a disease, are a promising tool for preventative medicine. However, they are often biased if trained only on European-ancestry populations. Federated learning is the only ethically and legally viable way to train and validate PRS on diverse, global data to ensure they are accurate and equitable for all populations.
- AI in Medical Imaging: Researchers can train deep learning models on medical images (e.g., digital pathology slides) from multiple hospitals to improve diagnostic accuracy for diseases like cancer. The federated model allows the algorithm to learn from all the images without the sensitive data ever leaving the protection of each hospital’s firewall.
Real-World Examples: How technology partners for large cohort studies like CanPath are accelerating discoveries
These are not theoretical capabilities. The CanPath and Lifebit partnership exemplifies how technology partners for large cohort studies like canpath accelerate research. Funded by Genome Canada’s Genomic Applications Partnership Program (GAPP), the project’s goal is to deploy a unified, federated analytics platform over CanPath’s rich data. This removes traditional barriers, allowing approved researchers worldwide to securely analyze the complex interplay of genetics, environment, and lifestyle in cancer and chronic disease without needing their own supercomputers.
Our work with Genomics England demonstrates this power at scale. The federated TRE over the 100,000 Genomes Project data has enabled researchers to uncover new genetic markers for rare diseases and cancer, leading directly to over 2,000 new diagnoses for patients and informing the UK’s national Genomic Medicine Service. The partnership with Flatiron Health shows how this model works for real-world cancer data, enabling secure, cross-country comparisons of patient cohorts to understand treatment effectiveness. In Singapore, Synapxe uses our federated TRE technology to power their national precision medicine research infrastructure, securely linking clinical and genomic data across the healthcare system.
These partnerships prove that federated platforms, cloud infrastructure, and advanced analytics are actively accelerating cancer research, uncovering genetic markers, and providing comprehensive insights into population health.
The Tangible Impact: Faster Findies and Better Health Outcomes
The adoption of advanced technology has a profound impact on the pace of scientific findy and, ultimately, on global health.

From Years to Months: Slashing the Time to Scientific Insight
The most dramatic impact is speed. The old model involved months of waiting for data access approvals and transfers. A project could take over a year before analysis even began. With streamlined data access through federated TREs, approved researchers can start analyzing data within days.
This efficiency enables rapid hypothesis testing. Researchers can test an idea, see the results, and refine their approach in a fraction of the time. This agility leads to faster publication of findings and quicker translation to clinical practice. When we identify disease biomarkers faster, diagnostic tools and treatments can reach patients sooner. As highlighted in the Cohort Profile: The Ontario Health Study (OHS), accelerating the research cycle multiplies the value of these long-term studies.
Economic and Operational Wins for Cohort Studies
Beyond the science, there is a compelling financial story. Large cohort studies traditionally faced enormous infrastructure costs for servers, storage, and IT staff. Reduced infrastructure costs through cloud-based solutions change this equation. Studies pay only for the computing resources they use, shifting from capital to operational expenditure.
This optimized resource allocation frees up funding for more research, participant recruitment, or richer data collection. It also helps in attracting top research talent, who want to work with state-of-the-art tools. Offering cutting-edge federated platforms makes studies like CanPath magnets for the best minds in the field.
Furthermore, demonstrating robust data management with trusted federated technology strengthens applications for securing funding. Most importantly, these solutions ensure sustainable long-term operations. Cloud-based federated platforms provide a foundation that can scale and adapt for decades, ensuring that technology partners for large cohort studies like canpath are building for the future of findy.
Frequently Asked Questions about Technology for Cohort Studies
How do federated platforms handle data from different countries with different laws?
The solution is that the data never leaves its home jurisdiction. A federated platform brings the analysis to the data. This means research on European data happens within a GDPR-compliant environment, while analysis on US data adheres to HIPAA. Each dataset remains secure and governed by local laws. Only aggregated, non-identifiable results are shared across borders. This model respects data sovereignty by design, enabling secure international collaboration.
Do my researchers need advanced coding skills to use these platforms?
Not at all. Modern federated platforms are built for researchers with all levels of technical expertise. They fully support code-based analysis in R or Python for bioinformaticians, but they also offer user-friendly interfaces with low-code and no-code tools. This democratization of access means epidemiologists and clinicians can explore data and run analyses without needing to be programmers. When more minds can engage with the data, we get better science.
How is participant privacy and trust maintained?
Participant privacy is the foundation of our platform design. The ‘analysis to data’ model is the first line of defense, as raw data is never moved, copied, or downloaded. Beyond that, we enforce strict access controls, ensuring only approved researchers on ethically approved projects can enter the environment. Data undergoes rigorous de-identification, and every action is logged in full audit trails. This multi-layered approach, governed by robust ethical frameworks, actively maintains the trust that makes groundbreaking population health research possible.
Conclusion
The era of copying and shipping massive, sensitive health datasets is over. It’s too slow, too risky, and it no longer works for modern research. Technology partners for large cohort studies like canpath are driving a fundamental shift by bringing analysis to the data.
Federated platforms are breaking down the data silos, security risks, and collaboration barriers that have held back research for decades. This new model empowers researchers to open up the full potential of population health data, enabling seamless international collaboration and AI-driven analysis across distributed datasets. The result is real breakthroughs: diseases are better understood, diagnostics become more accurate, and treatments improve.
At Lifebit, we have spent over 15 years building the federated AI platform to make this vision a reality for the world’s most ambitious health initiatives, from Genomics England to CanPath. We’ve seen how the right technology doesn’t just support research; it transforms it.
The future of population health research is federated, secure, and collaborative. It’s a future where researchers spend less time on bureaucracy and more time making findies that improve human health.
Ready to see what this means for your research? Learn more about solutions for government and public health.

