The Future is Now for Clinical Trial Technology

88% of Drugs Fail in Trials: How Technology is Reversing a Decade of Failure
Clinical trial technology is the use of digital tools—like remote monitoring, AI analytics, wearable sensors, and cloud platforms—to design, run, and analyze medical research studies faster, cheaper, and with better data quality than traditional paper-based or site-only approaches.
Key components of clinical trial technology include:
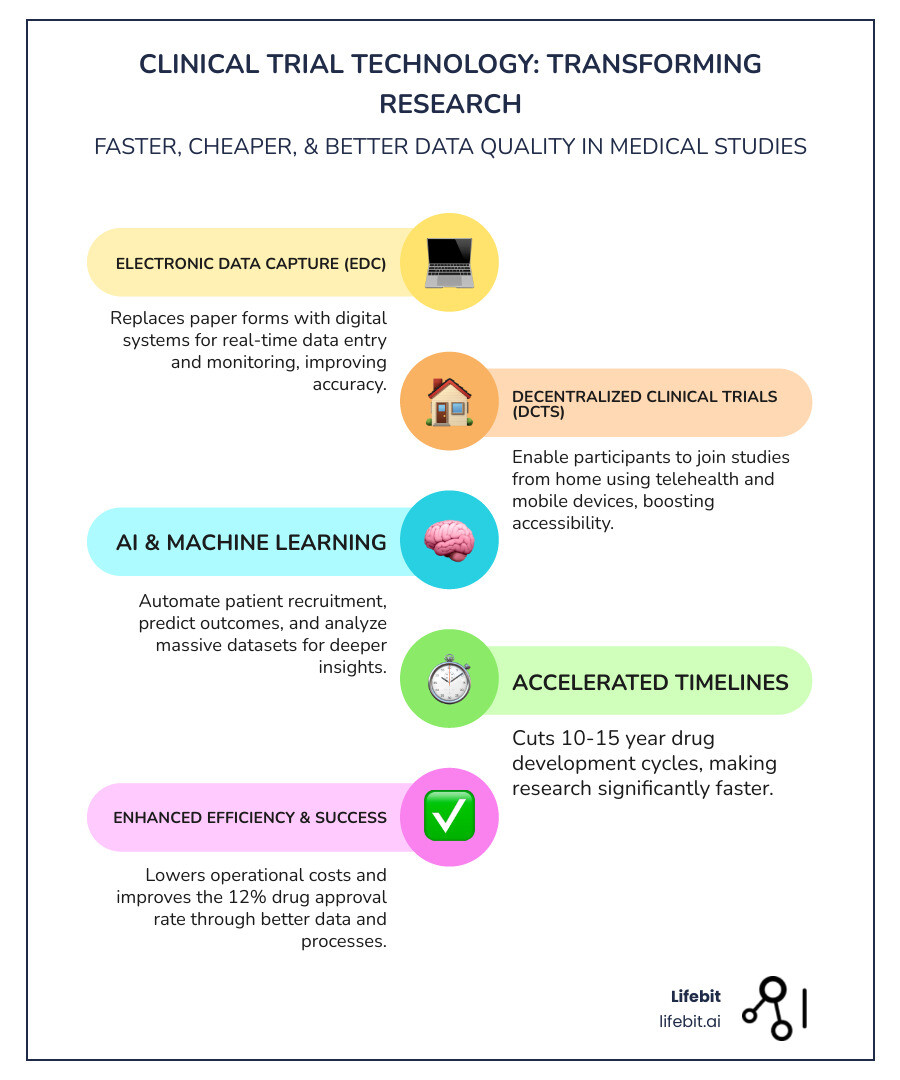
- Electronic Data Capture (EDC) – Replaces paper forms with digital systems for real-time data entry and monitoring
- Decentralized Clinical Trials (DCTs) – Enable participants to join studies from home using telehealth and mobile devices
- Digital Health Technologies (DHTs) – Wearables, apps, and sensors that collect continuous health data remotely
- AI and Machine Learning – Automate patient recruitment, predict outcomes, and analyze massive datasets
- Clinical Metadata Repositories (CMDRs) – Standardize and manage study metadata for faster setup and regulatory compliance
The urgency is real. Traditional clinical trials take 10 to 15 years to complete all three phases before licensing, and only about 12 percent of drugs entering trials ever get FDA approval. The system is too slow, too expensive, and too burdensome for patients—especially when lives hang in the balance.
The COVID-19 pandemic changed everything. Weekly virtual healthcare visits for Medicare beneficiaries surged from 13,000 before the pandemic to 1.7 million in April 2020. Researchers proved that trials could move faster without sacrificing safety or data quality. A randomized trial evaluating hydroxychloroquine was launched, enrolled, and published—entirely electronically—within 90 days. The Metastatic Breast Cancer Project enrolled nearly 3,000 volunteers in its first year using social media, online consent, and remote data collection. These weren’t one-off experiments. They were proof that the old model was broken—and that technology could fix it.
Today, clinical trial technology is no longer optional. It’s how we bring treatments to patients faster, make trials more accessible to diverse populations, and finally harness the power of real-time data. The tools exist. The regulatory pathways are clearing. The question is no longer if but how quickly organizations will adopt them.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve spent over 15 years building platforms that enable secure, federated access to global health data—powering clinical trial technology that lets researchers analyze siloed datasets in real time without moving sensitive information. My work has focused on making precision medicine scalable, compliant, and accessible to every institution that needs it, from pharma giants to public health agencies.
Clinical trial technology terms you need:
Slash Timelines and Costs: How Clinical Trial Technology is Revolutionizing Research
The traditional clinical trial model has long been a bottleneck in medical progress. Imagine a process so complex and drawn-out that it takes 10 to 15 years or more to complete just the three phases before a drug can even be licensed. And despite this immense investment of time and resources, only about 12 percent of drugs entering clinical trials are ultimately approved for introduction. This isn’t just inefficient; it’s a critical barrier to delivering life-saving treatments to patients in need. We are committed to changing this narrative.
Clinical trial technology is changing medical research by making it faster, more efficient, and ultimately more successful. Our approach leverages digital tools to accelerate data collection and analysis, leading to faster treatment delivery. This change allows us to:
- Reduce Drug Development Time: By streamlining processes that traditionally took months or even years, such as data entry and site management, we can significantly cut down the overall timeline for clinical trials.
- Lower Operational Costs: Manual, paper-based processes are not only slow but also incredibly expensive. Clinical trial technology automates many of these tasks, reducing the need for extensive human resources and physical infrastructure.
- Improve Data Quality and Accuracy: Digital systems minimize human error inherent in manual data handling. They also allow for real-time data monitoring and immediate identification of discrepancies, ensuring that the data collected is robust and reliable.
- Improve Trial Success Rates: With better data, faster insights, and more agile trial designs, we can make quicker, more informed decisions, improving the likelihood of a trial’s success.
The shift is akin to upgrading from a flip phone to a smartphone. The impact on speed and effectiveness is profound. As one expert insight highlights, digital tools accelerate data collection and analysis, leading to faster treatment delivery. Furthermore, advanced capabilities like AI and machine learning expedite data analysis beyond human capabilities in speed and accuracy. This allows us to move from simply gathering data to truly understanding it, driving progress in ways previously unimaginable.
Automation plays a crucial role in this streamlining. It handles extensive datasets quickly and accurately, allowing researchers to focus on analysis rather than tedious data management. This not only speeds up the entire process but also significantly reduces the potential for human error. We believe that by embracing these technological advancements, we can overcome the historical challenges of drug development and bring innovative therapies to patients much more rapidly.
Ditch the Paperwork: 3 Technologies Now Driving Trial Efficiency
Clinical research sites often face a multitude of challenges that can hinder the progress of a trial. These common pain points include busy, overworked staff, difficulties with patient recruitment and timely visits, the burden of countless pre-recruitment activities, and the time-consuming tasks of data entry, data cleaning, and quality control. These administrative burdens and inefficient workflows can lead to burnout among clinical research coordinators and delay crucial research.
Clinical trial technology offers powerful solutions to these issues, driving efficiency and changing how trials are conducted. Let’s explore the key technologies in our arsenal.
The Power of Electronic Data Capture (EDC) and Automation
Electronic Data Capture (EDC) systems are fundamental to modern clinical trial technology. They replace traditional paper forms with digital interfaces, allowing for direct, real-time data entry and monitoring. This immediately cuts down on paper-based logistics and the errors associated with manual transcription. The evolution of these systems has been rapid, moving from simple web-based forms to sophisticated, integrated platforms.
But EDC systems are just the beginning. The real power comes when they are integrated with other technologies to create a unified clinical trial ecosystem. EHR-to-EDC integration, for instance, allows for seamless and automated transfer of relevant patient data from Electronic Health Records (EHR) directly into the EDC system. Standards like HL7 FHIR are critical here, enabling interoperability and reducing the manual, time-consuming nature of chart abstraction. This integration significantly impacts site workload by:
- Reducing Manual Data Entry: Site staff spend less time on repetitive data input, freeing them for more patient-focused activities and complex problem-solving.
- Providing Real-Time Data Access: Data is immediately available for review and analysis, enabling quicker decision-making and early identification of trends or issues. This supports modern methodologies like Risk-Based Quality Management (RBQM), where monitoring efforts are focused on the most critical data and processes.
- Improving Data Provenance: As one user highlighted, modern platforms enable us to “follow the provenance of data, from point of collection all the way to monitoring, tracking, and reporting.” This transparency and traceability are vital for data quality and regulatory compliance.
Automation extends beyond just data capture. When an EDC is part of a unified platform that includes a Clinical Trial Management System (CTMS), automation can orchestrate the entire trial. This includes streamlining site payments, managing study and site documentation, tracking patient visit schedules, and automating task assignments. For example, direct data entry and real-time data cleaning reduce the time spent on resolving queries, which traditionally could take weeks. This comprehensive automation minimizes human error, improves data accuracy, and drastically boosts efficiency across the entire trial lifecycle, creating a single source of truth for all trial-related information.
Decentralized Clinical Trials (DCTs) and Digital Health Technologies (DHTs)
The concept of Decentralized Clinical Trials (DCTs) is perhaps the most transformative aspect of modern clinical trial technology. DCTs bring the trial to the patient, rather than requiring patients to travel to distant research sites. This is largely enabled by a diverse array of Digital Health Technologies (DHTs), which include:
- Wearables and Sensors: These devices, such as smartwatches or specialized biosensors, can continuously collect physiological data like heart rate, sleep patterns, activity levels, and even specific disease indicators. For example, in an epilepsy study, the measurement of daily seizure frequency between an app and paper diaries showed high concordance at 93.0 percent and 88.2 percent for two study arms, demonstrating the reliability of digital tools for objective data collection. We have seen that continuous monitoring can provide richer efficacy and safety information than traditional episodic data capture. Other examples include smart inhalers for asthma trials that track usage and technique, or continuous glucose monitors in diabetes studies.
- Mobile Health (mHealth) Apps: Smartphone and tablet applications allow participants to report symptoms (ePRO), receive medication reminders, complete surveys, and engage with the study team remotely. With about 8 billion mobile phone subscriptions worldwide and more than half of people over 65 years of age owning a smartphone, these tools are highly accessible across diverse populations. Some apps even use the phone’s camera for dermatological assessments or to monitor medication intake (e.g., digital pills).
- Telehealth Visits: Virtual consultations via video conferencing replace many in-person visits, offering convenience and reducing travel time and costs for participants.
- DCT Logistics and Supply Chain Tech: A crucial, often overlooked component is the technology managing the logistics of a decentralized trial. This includes platforms for coordinating direct-to-patient (DtP) shipments of investigational products, managing home nursing visits for procedures like blood draws, and ensuring the cold chain for sensitive materials is maintained all the way to the patient’s home.
The significance of DCTs and DHTs lies in their ability to make clinical research more patient-centric. They reduce patient burden by eliminating the need for frequent travel and allowing participants to engage with trials from the comfort of their homes. This expanded access is particularly crucial for individuals in remote areas, those with limited mobility, or patients with rare diseases. As we learn from implementing digital technologies in clinical trials, providing appropriate training and infrastructure support is key to addressing challenges and opening up the full potential of these transformative tools.
The Role of AI and Predictive Analytics in clinical trial technology
Artificial Intelligence (AI) and machine learning are rapidly becoming indispensable components of clinical trial technology, propelling efficiency and insight generation to new levels. Our federated AI platform, for instance, enables secure and real-time access to vast biomedical datasets, allowing for advanced analytics without compromising data privacy.
AI and predictive analytics contribute significantly by:
- AI in Patient Recruitment: AI algorithms can analyze real-world data, including electronic health records and demographic information, to identify potential trial participants more effectively. Using Natural Language Processing (NLP), AI can scan unstructured clinical notes to find patients who meet complex eligibility criteria that structured data alone would miss. This allows us to target recruitment efforts, ensuring a more diverse and representative participant pool.
- Predictive Modeling for Trial Outcomes: By analyzing historical data, AI can predict the likelihood of success for different trial designs or identify potential roadblocks. An emerging application is the creation of ‘synthetic control arms,’ where AI models use real-world data from past patients to simulate a placebo group, potentially reducing the need to recruit as many control patients in future trials.
- AI-Powered Data Analysis: AI and machine learning algorithms can process and interpret massive, complex datasets generated in clinical trials far more rapidly and accurately than human analysts. This includes automating tedious tasks like medical coding, where AI can suggest appropriate MedDRA codes for adverse events or WHODrug codes for medications, ensuring consistency and saving significant time. This speeds up the identification of patterns, insights, and potential safety signals.
- Identifying Potential Risks Early: AI can continuously monitor incoming data for anomalies or adverse event trends, flagging them for immediate investigation and intervention. This is a cornerstone of modern risk-based monitoring, as AI can detect subtle patterns across thousands of data points that would be invisible to a human reviewer, potentially indicating site-level issues or data fraud.
- Streamlining Protocol Design: Leveraging AI to analyze previous trial designs and outcomes can help optimize new protocols, ensuring they are robust, efficient, and patient-centric from the outset. This efficiency is remarkable; we’ve seen instances where “one team member built three unique studies in three months, from protocol to go-live,” highlighting the power of intelligent automation and standardized metadata.
The integration of AI into clinical trial technology transforms research from a reactive process to a proactive, data-driven endeavor, leading to faster, more effective, and ultimately more successful trials.
From Burden to Partnership: How Technology Transforms the Patient Experience
For too long, participating in a clinical trial has been an arduous journey for patients, often involving frequent, inconvenient site visits, significant travel costs, and time away from work or family. This burden contributes to low recruitment rates and high dropout rates. Clinical trial technology is fundamentally shifting this dynamic, changing the patient experience from a burden into a true partnership.
We are seeing significant improvements in patient recruitment and retention through various digital innovations:
- Improved Patient Recruitment: Online platforms and social media engagement have revolutionized how we connect with potential participants. The Metastatic Breast Cancer Project, for example, successfully leveraged these channels to enroll nearly 3,000 volunteers in its first year, showcasing the power of digital outreach.
- Improved Patient Retention: By reducing the logistical and financial barriers to participation, technology helps keep patients engaged. Remote monitoring and telehealth visits mean fewer trips to the clinic, allowing participants to integrate the trial into their daily lives with minimal disruption.
- eConsent (Electronic Informed Consent): Moving beyond stacks of paper, eConsent uses digital platforms with interactive elements and educational videos to make the consent process more understandable and accessible. This not only streamlines the process but also ensures participants are better informed about the study.
- Gamification for Engagement: Integrating game-like elements into study apps can make participation more engaging and encourage adherence to protocols, especially in long-term studies.
- Reduced Travel and Financial Burden: This is perhaps one of the most significant benefits for patients. Remote data collection and virtual visits eliminate the need for costly and time-consuming travel, which can be a major deterrent for many, particularly those in rural areas or with limited resources.
- Increased Trial Diversity and Access: By removing geographical and logistical barriers, clinical trial technology opens up participation to a much broader and more diverse demographic. This is crucial for ensuring that new treatments are effective across all populations. For instance, studies conducted solely via telemedicine have successfully enrolled participants from numerous regions, proving the expansive reach of remote trials. With more than half of people over 65 owning a smartphone, the digital divide is narrowing, making these technologies accessible to a wider age range.
By placing the patient at the center of trial design and leveraging technology to improve convenience and engagement, we foster a more inclusive and effective research environment.
The 6 Roadblocks to Clinical Tech Adoption (And How to Overcome Them)
While the potential of clinical trial technology is immense, its widespread adoption isn’t without problems. We recognize that implementing new digital solutions in a highly regulated and complex environment like clinical research presents several challenges:
- Data Privacy and Security: The collection of sensitive patient health information through various digital devices and platforms raises significant concerns about data privacy and cybersecurity. Ensuring compliance with regulations like GDPR and HIPAA, and building robust security frameworks, is paramount. This involves end-to-end encryption, multi-factor authentication, strict access controls, and thorough de-identification or pseudonymization of data. Our federated approach, for example, allows for analysis of data without physically moving it—models are sent to the data, and only aggregated, non-identifiable results return—significantly enhancing security and maintaining data sovereignty.
- Regulatory and Compliance Problems: The rapid pace of technological innovation can outstrip regulatory frameworks. Ensuring that novel technologies and decentralized trial designs meet stringent regulatory requirements (like those from the FDA or EMA) can be complex. For instance, all electronic systems must comply with standards like FDA 21 CFR Part 11, which governs electronic records and signatures. A major hurdle is the validation of novel digital endpoints; sponsors must prove to regulators that a metric from a wearable is a reliable and clinically meaningful measure of a disease state. This requires proactive engagement with authorities and a clear validation strategy.
- Digital Literacy Gaps: While smartphone ownership is high, not all participants or even site staff have the same level of digital proficiency. This can lead to issues with device usability, data input, and overall engagement. Overcoming this requires a user-centric design approach, involving patients and sites in the development process. Practical solutions include providing pre-configured devices, offering simple onboarding tutorials, and establishing a 24/7 multilingual helpdesk. It’s also critical to address the ‘tech burden’ on sites, which are often forced to use dozens of different, non-integrated platforms for various trials, each with its own login and workflow.
- Interoperability and Integration Issues: Clinical research often involves a patchwork of different systems—EHRs, EDCs, lab systems, ePRO apps, and various DHTs. Making these disparate systems communicate seamlessly and exchange data effectively is a major technical challenge. Without robust interoperability, enabled by modern Application Programming Interfaces (APIs) and data standards, the promise of streamlined workflows remains unfulfilled. For example, if an ePRO app doesn’t sync with the EDC, a site coordinator must manually transcribe patient-reported outcomes, reintroducing human error and negating the technology’s benefit.
- Data Standardization Needs: The data collected from diverse sources and technologies needs to be standardized and normalized to be comparable and interpretable. This is where the significance of metadata standardization truly comes into play. Clinical Metadata Repositories (CMDRs) are essential tools that provide a centralized, reusable library for storing and managing all the definitions that make up a study—from visit schedules and forms to specific questions and code lists. This drastically reduces the time spent on creating and approving metadata for each new study and ensures that data conforms to industry standards like those from CDISC (e.g., SDTM, ADaM), which is critical for regulatory submissions and cross-study analysis.
- Ensuring Data Quality from Novel Sources: Data from wearables and sensors can be continuous and rich, but it also presents challenges like differentiating “signal from noise” and managing potential artifacts (e.g., a heart rate spike caused by a dropped device). Rigorous validation and careful data curation are needed to ensure the quality and reliability of these novel data streams. This often follows a V3 framework (Verification, Analytical Validation, Clinical Validation) to establish a new digital biomarker. For example, to use a smartphone’s accelerometer to measure gait changes in Parkinson’s disease, one must first verify the sensor’s accuracy, then analytically validate the algorithm that calculates gait, and finally clinically validate that the resulting metric correlates with established clinical scales.
Addressing these challenges requires a multi-faceted approach, combining technological innovation with careful planning, robust security, and ongoing collaboration with regulatory bodies and stakeholders.
Beyond Decentralized Trials: A 5-Year Look into the Future of Clinical Tech
The landscape of clinical research is undergoing a profound change, driven by the relentless march of clinical trial technology. Looking ahead, we foresee a future where digital health solutions fundamentally reshape trial design and operations, making them more agile, insightful, and ultimately, more impactful.
Here’s a glimpse into what’s next:
| Feature | Traditional Trials | Fully Decentralized Trials |
|---|---|---|
| Location | Centralized physical sites | Patient’s home, local clinics, remote |
| Data Collection | Manual, episodic, paper CRFs | Electronic, continuous, DHTs, EHR-to-EDC |
| Patient Burden | High (travel, time, costs) | Low (convenience, reduced travel) |
| Recruitment | Limited by geography | Wider reach, diverse populations |
| Monitoring | On-site visits, source data verification | Remote, centralized, AI-driven anomaly detection |
| Timeline | Long (10-15+ years) | Accelerated (real-time data, agile design) |
| Cost | High (site overheads, travel reimbursement) | Potentially lower (reduced site visits, optimized resources) |
- Real-World Evidence (RWE) Integration: The future will increasingly leverage Real-World Evidence (RWE) derived from electronic health records, claims data, and patient registries to inform clinical trial design, identify eligible patients, and even support regulatory decision-making. This moves beyond traditional clinical trial data to provide a more holistic view of drug efficacy and safety in diverse patient populations.
- The Rise of Virtual-First Trials: While hybrid models will remain prevalent, we anticipate a growing number of “virtual-first” or even fully virtual trials, particularly for certain conditions or therapeutic areas. This approach maximizes patient convenience and broadens access, especially in regions with limited research infrastructure.
- Hyper-Personalized Medicine: Advanced analytics, including AI and machine learning, combined with multi-omic data (genomics, proteomics, metabolomics), will enable us to design trials that are custom to individual patient profiles. This hyper-personalization will lead to more targeted therapies and more effective treatments. Our federated AI platform is designed to securely access and analyze such complex, multi-omic datasets without moving sensitive information.
- Blockchain for Data Integrity: Blockchain technology holds significant promise for enhancing the security, transparency, and immutability of clinical trial data. By creating an unchangeable record of all trial activities and data entries, blockchain can bolster trust and streamline auditing processes.
- The Evolving Role of Regulatory Bodies: Regulatory agencies like the FDA are actively adapting to these changes. Their guidance on Digital Health Technologies and Decentralized Clinical Trials provides a roadmap for safe and effective implementation, ensuring that innovation doesn’t compromise patient safety or data integrity. We anticipate continued collaboration between technology providers, researchers, and regulators to shape the future of clinical research.
The clinical trial technology research team of the future will be multidisciplinary, including computer scientists and engineers as critical partners, alongside clinicians and statisticians. This collaborative approach will be essential to harness the full potential of these transformative technologies.
Frequently Asked Questions about Clinical Trial Technology
What are decentralized clinical trials (DCTs)?
Decentralized Clinical Trials (DCTs) are a modern approach to medical research that incorporates elements allowing trial-related activities to occur remotely, often at locations convenient for the participant rather than solely at a traditional clinical site. This can range from hybrid models, where some visits are remote and others are on-site, to fully virtual trials. DCTs use digital health technologies like wearables, mobile apps, and telehealth to enable remote data collection, monitoring, and communication. They can involve local labs for routine tests or home health services for procedures like blood draws. The benefits for patients include improved convenience, reduced travel burden, and increased access to studies. For sponsors, DCTs can expand recruitment to more diverse populations and improve trial efficiencies.
How does technology improve data quality in clinical trials?
Clinical trial technology significantly improves data quality by reducing human error, enabling real-time monitoring, and enforcing standardization. Automated data capture via EDC systems eliminates manual transcription, a common source of errors. When EHR-to-EDC integration is implemented, data flows directly from clinical records, further minimizing manual input. Real-time monitoring allows for immediate identification and resolution of data discrepancies or queries, rather than finding them weeks or months later. Furthermore, digital systems facilitate the use of standardized data formats (like CDISC), ensuring consistency across studies and sites. Continuous data streams from wearables and sensors provide objective, granular information that is less prone to recall bias than self-reported data, offering a more complete and accurate picture of patient health.
Are wearables and sensors reliable for collecting clinical data?
Yes, wearables and sensors can be highly reliable for collecting clinical data, provided they undergo rigorous validation. While consumer-grade devices offer broad accessibility, clinical trials often require devices with specific validation for their intended use, sometimes even FDA clearance. The benefits are substantial: they offer objective, continuous data that can capture subtle changes or infrequent events that might be missed during episodic clinic visits. For example, a study showed high concordance (over 88%) between an app and paper diaries for daily seizure frequency, demonstrating their reliability. However, challenges include differentiating signal from noise in large datasets, managing data artifacts, and ensuring proper device usage by participants. Careful selection of devices, thorough validation, and comprehensive training for both participants and staff are crucial to using their reliability effectively.
Conclusion: Building the Future of Medicine, Together
The journey of drug development has historically been long, expensive, and often inaccessible to many patients. However, the advent of clinical trial technology has heralded a new era, promising a future where medical research is faster, more cost-effective, and profoundly more patient-centric. We have seen how digital tools, from advanced EDC systems and AI analytics to decentralized trial models and innovative digital health technologies like wearables, are dismantling traditional barriers and accelerating the path to new treatments.
The shift is permanent. The lessons learned, particularly from the rapid adaptations during the COVID-19 pandemic, have underscored the immense potential of these technologies. We now have the tools and the collective experience to conduct trials that are not only more efficient but also more inclusive and reflective of real-world patient populations.
At Lifebit, we are proud to be at the forefront of this revolution. Our secure federated AI platform is specifically designed to power this next generation of research. By enabling secure, real-time access to global biomedical and multi-omic data, we empower biopharma, governments, and public health agencies to conduct compliant, large-scale research. Our built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance ensure that researchers can gain real-time insights and drive AI-driven safety surveillance across diverse and often siloed hybrid data ecosystems, all without compromising data privacy. Together, we are not just adopting technology; we are building the future of medicine.

