Why Your Clinical Trial Data and EHRs Should Be Best Friends

How Secure Data Linkage Between Clinical Trial Data and EHR Cuts Redundancy by 70%
Secure data linkage between clinical trial data and EHR solves a massive inefficiency: approximately 70% of the data entered into Electronic Data Capture (EDC) systems is duplicated from Electronic Health Records (EHRs) and other source systems. This manual re-entry wastes time, increases errors, and burns out site coordinators—all while slowing down the pace of medical breakthroughs.
What you’ll learn in this guide:
- Why linkage matters: How connecting trial data to EHRs reduces costs, speeds completion, and improves data quality
- Core technologies: Common Data Elements (CDEs), tokenization, and Privacy-Preserving Record Linkage (PPRL)
- Real-world impact: How EHR integration enables long-term follow-up, mitigates loss to follow-up (LTFU), and supports regulatory submissions
- Implementation steps: Patient consent, site training, honest broker systems, and evaluation metrics
- Future innovations: AI-driven connectors, federated platforms, and global scalability
Right now, clinical research and patient care operate in separate silos. A hospital patient enrolling in a Phase 3 trial might have their smoking history already documented in the EHR—but the research nurse still has to re-collect that same information manually because the systems don’t talk to each other. When a physician notes a headache informally, it may never connect to the trial’s adverse event requirements. This separation makes clinical research unnecessarily expensive and time-consuming, and it blocks the vision of a “Learning Health System” where every patient encounter contributes to scientific knowledge.
The good news? Industry changes like the 21st Century Cures Act, TEFCA (Trusted Exchange Framework and Common Agreement), and the adoption of HL7 FHIR standards have finally created an “interoperability floor.” Advanced interoperability platforms now enable seamless EHR-to-EDC integration—some achieving up to 90% faster form completion than manual entry. Meanwhile, privacy-preserving technologies like tokenization allow trial participants to be securely linked to real-world data sources (claims, registries, EHRs) for long-term follow-up—without ever exposing Protected Health Information (PHI).
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built federated AI platforms that power secure data linkage between clinical trial data and EHR systems for over 275 million patient records globally. Throughout this guide, I’ll show you how to implement these technologies at scale—and why they’re essential for the future of clinical research.
Secure data linkage between clinical trial data and ehr helpful reading:
The High Cost of Data Silos: Why Secure Data Linkage Between Clinical Trial Data and EHR is Essential
The wall between clinical care and clinical research is more than just an administrative hurdle—it is a financial and operational drain. When we look at the numbers, the status quo is staggering. Research indicates that 70% of the data manually typed into EDC systems during a trial already exists within the patient’s EHR. This redundancy leads to a “double-entry” nightmare for site research coordinators, contributing significantly to burnout and high turnover rates at clinical sites.
The Economic Burden of Manual Data Entry
Beyond the human cost, the financial implications are profound. Source Data Verification (SDV)—the process where a Clinical Research Associate (CRA) physically visits a site to compare EDC entries against the original EHR records—can consume up to 25-30% of a total clinical trial budget. In a large-scale Phase 3 trial, this translates to millions of dollars spent simply on verifying that data was copied correctly from one screen to another. By establishing secure data linkage between clinical trial data and EHR, we can move toward “Remote Source Data Verification” or even eliminate the need for manual SDV entirely through automated, validated data transfers.
The Fragmentation Problem and the Learning Health System
Data fragmentation is the “silent killer” of trial efficiency. Currently, a single patient’s journey is scattered across primary care EHRs, specialist portals, pharmacy claims, and hospital registries. If a trial participant moves or changes insurance, they are often marked as “Lost to Follow-Up” (LTFU). This missing data can bias trial results and weaken the statistical power of a study. This separation blocks the vision of a “Learning Health System,” envisioned by the Institute of Medicine, where research is naturally integrated into patient care. As noted in scientific research on EHR integration challenges, the primary purpose of clinician documentation remains patient care. Without automated linkage, research needs—like granular smoking history or specific adverse event coding—often get lost in the shuffle.
By establishing secure linkage, we can:
- Reduce Trial Costs: Automated data transfers eliminate the need for expensive, manual SDV.
- Gain Real-Time Insights: Instead of waiting months for data “cleaning,” sponsors can monitor safety signals as they happen.
- Achieve a Nationwide Health System: Linkage supports the broader goal of achieving a nationwide learning health system where data flows securely to improve outcomes for all.
Solving the Interoperability Puzzle with CDEs and Tokenization
If linkage is the goal, interoperability is the engine. However, EHRs and Clinical Trial Management Systems (CTMS) speak different languages. EHRs are designed for unplanned, single-patient care, while trial systems are built around rigid, protocol-based research tasks. To bridge this gap, we use Common Data Elements (CDEs). These are standardized variables—such as “Systolic Blood Pressure” or “HbA1c Level”—that have fixed definitions and metadata. The National Library of Medicine CDE trends show a growing movement toward using these elements to ensure that data captured in a clinic can be “computable” for a trial without manual re-mapping.
The Technical Rails: HL7 FHIR and APIs
Standards like HL7 FHIR (Fast Healthcare Interoperability Resources) provide the technical rails for this data to move. FHIR uses “Resources” (like Patient, Observation, Condition, and MedicationStatement) to package data in a way that any system can understand. Furthermore, regulatory mandates like the 21st Century Cures Act and TEFCA have pushed EHR vendors to open their systems via APIs, finally making harmonizing disparate electronic health records a reality. Standardized phenotyping and research on metadata registries ensure that when we say “Type 2 Diabetes,” the research system and the EHR are in total agreement.
How Tokenization Powers Secure Data Linkage Between Clinical Trial Data and EHR
The biggest fear in data linkage is the exposure of Protected Health Information (PHI). This is where Privacy-Preserving Record Linkage (PPRL) and tokenization come in. Tokenization is a process that replaces sensitive identifiers (like Name, DOB, or SSN) with a unique, encrypted string called a “token.” This is done using a one-way cryptographic hash function—meaning the process cannot be reversed to reveal the original identity.
- Hashing: Identifiers are passed through an algorithm (like FIPS 140-2). This creates a complex alphanumeric string that represents the patient’s identity without containing any readable data.
- Salting: A secret “salt” key is added to the hash. This is a critical security step; it ensures that even if two different projects use the same hashing algorithm, the resulting tokens will be different, preventing unauthorized cross-referencing of data.
- Matching: These tokens are then used to find the same patient across different datasets (e.g., matching a trial participant to their pharmacy claims) without any party ever seeing the patient’s name.
According to research on PPRL performance evaluation, these systems can achieve precision rates of over 99%. This allows us to create a patient-centric linkage that follows the individual across the healthcare ecosystem while keeping their identity completely locked away.

Real-World Impact: From Long-Term Follow-Up to Regulatory Success
The most immediate benefit of secure data linkage between clinical trial data and EHR is the ability to track long-term outcomes. Many modern therapies, such as CAR-T cell treatments or gene therapies for rare diseases, require monitoring for 5, 10, or even 15 years. It is operationally impossible—and prohibitively expensive—to keep traditional trial sites open for that long. By linking trial participants to their EHR and claims data, we can “passively” monitor for late-occurring adverse events or sustained efficacy. This is critical because, as research on treatment effects and missing data shows, high rates of loss to follow-up can completely invalidate a study’s findings.
Regulatory-Grade Evidence and Synthetic Control Arms
Regulators like the FDA and EMA are increasingly accepting Real-World Evidence (RWE) to support new drug indications or post-market safety requirements. The FDA guidance on RWD for regulatory decisions provides a framework for using EHR and claims data to support drug and biological product approvals. One of the most exciting applications is the creation of Synthetic Control Arms (SCA). By using linked EHR data from patients receiving the standard of care in the real world, sponsors can reduce the number of patients required for a placebo group in a trial. This not only speeds up the trial but also makes it more ethical, as more participants can receive the experimental therapy.
Key applications include:
- Generalizability and Transportability: Using RWD to see if trial results hold true in broader, more diverse populations (e.g., different ethnicities or age groups) that are often underrepresented in traditional trials.
- Validation Substudies: Comparing EHR variables against trial “gold standards” to prove the RWD is high-quality and reliable for regulatory submission.
- Safety Surveillance: Detecting rare side effects that only appear in thousands of patients over several years, which a standard 12-month trial would never catch.
Maximizing ROI Through Secure Data Linkage
The return on investment (ROI) for linkage is clear. EHR-to-EDC integration solutions can enable site staff to complete forms up to 90% faster than manual entry. This speed allows trials to reach their “Last Patient Out” milestone months earlier, potentially saving millions in operational costs. However, technology alone isn’t enough. Successful linkage requires Honest Brokers—trusted third-party systems to manage the de-identification process—and Site Training to ensure Clinical Research Coordinators (CRCs) know how to use “smart templates” that prompt for research-quality data during routine visits. As shown in a case study on RCT-claims linkage, nearly 50% of trial patients can be successfully matched to claims databases, providing a massive boost to longitudinal data.
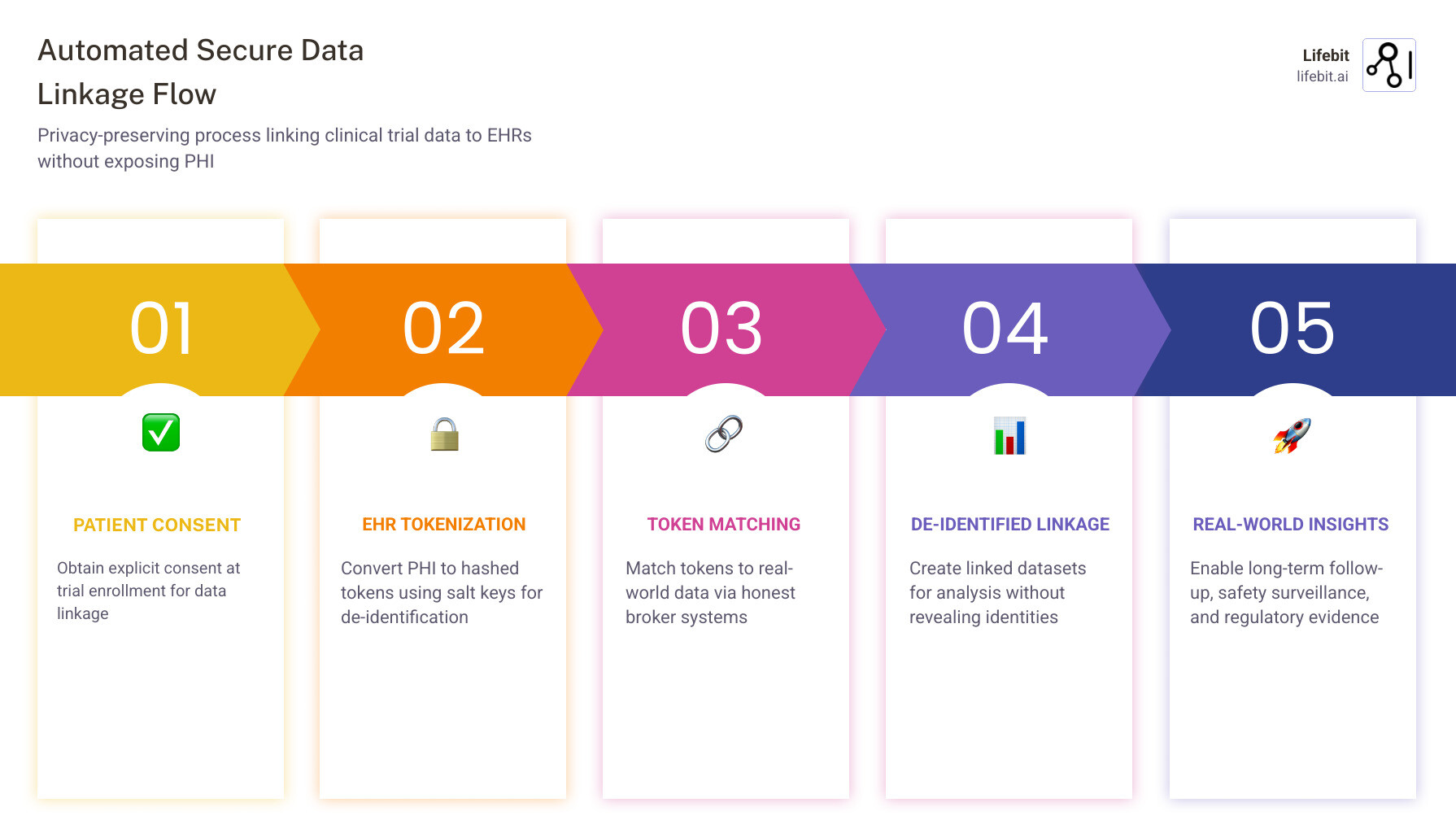
Operationalizing the Linkage: Steps for Scalable Implementation
Moving from a pilot to a global, scalable linkage program requires a structured approach. At Lifebit, we advocate for a federated architecture where data stays at its source (the hospital or clinic), and only the insights move. This respects local data sovereignty and simplifies compliance with laws like GDPR and HIPAA.
Step 1: Patient Consent and Transparency
Linkage must begin with a transparent consent process. Participants should be informed that their de-identified data will be used for long-term follow-up. Using digital consent management allows patients to opt-in or opt-out of specific data sources (e.g., linking to pharmacy records but not genomic registries). This builds trust and ensures ethical compliance.
Step 2: Site “Phenotyping” and Readiness Assessment
Not all sites are ready for direct EHR integration. You must assess site-level IT resources. Some sites might use “Central Change,” where they send de-identified flat files to a coordinating center, while others might use “Network Consortium” strategies where data is already mapped to a Common Data Model like OMOP. A site readiness checklist should include:
- Availability of FHIR-enabled APIs
- Staff capacity for technical training
- Legal clearance for data sharing agreements
Step 3: Choosing a Matching Strategy
When linking records, you must decide between deterministic and probabilistic matching.
| Feature | Deterministic Matching | Probabilistic Matching |
|---|---|---|
| Method | Exact match on specific fields (e.g., SSN). | Uses weights and scores to find “likely” matches. |
| Accuracy | Very high (low false positives). | Good for messy data with typos or missing fields. |
| Recall | Lower (misses people with minor data errors). | Higher (captures more matches). |
| Best Use | When high-quality identifiers are available. | When linking to older registries or claims data. |
Step 4: AI-Driven Connectors and Data Governance
The future of linkage lies in AI-driven connectors that can read unstructured clinical notes and map them to research standards automatically. This mitigates the gaps in structured EHR data and helps overcome “claims sparsity.” Furthermore, robust Data Governance is essential. This involves establishing clear protocols for who can access the linked data, how long it is stored, and the specific research questions it is intended to answer. Regular audits and quality assurance checks ensure that the linked dataset remains a “source of truth” for regulatory bodies.
Frequently Asked Questions about EHR-Trial Integration
What are the main barriers to EHR-EDC interoperability?
The primary barriers are differing data models, proprietary vendor systems, and a lack of standardized documentation in routine care. While technical standards like FHIR are helping, the human element—such as site staff overestimating their IT capabilities—remains a challenge. Centralized support and flexible extraction strategies are essential to overcome these problems.
How does tokenization protect patient privacy without exposing PHI?
Tokenization uses “one-way” cryptographic hashing. It takes a patient’s identifiers and turns them into a string of characters that cannot be “cracked” back into a name. By using unique “salts” for different projects, we ensure that tokens cannot be used to re-identify patients across unauthorized datasets, meeting the HIPAA Expert Determination standard for de-identification.
Can EHR data effectively mitigate loss to follow-up in long-term trials?
Yes! By linking trial participants to national claims databases or regional EHR networks, sponsors can track major health events (hospitalizations, deaths, or new diagnoses) even if the patient stops visiting the trial site. This “passive” follow-up ensures that the trial’s final analysis is based on a complete picture of the patient’s health journey.
What is the difference between data harmonization and data linkage?
Data harmonization is the process of making disparate datasets compatible by mapping them to a common standard (like OMOP or CDISC). Data linkage is the process of connecting records that belong to the same individual across those different datasets. You often need harmonization to make the linkage useful for analysis.
Is secure data linkage compliant with GDPR and international laws?
Yes, provided it is implemented correctly. Using a federated approach—where data is analyzed locally and only aggregated results are shared—minimizes data transfer across borders. Combined with robust de-identification and explicit patient consent, these systems are designed to meet the world’s strictest privacy regulations, including GDPR in Europe and HIPAA in the US.
Conclusion
The era of manual, siloed clinical research is ending. By embracing secure data linkage between clinical trial data and EHR, we can finally eliminate the 70% data redundancy that has plagued our industry for decades. Technologies like tokenization and federated AI allow us to open up the power of real-world data while maintaining the highest standards of patient privacy.
At Lifebit, we believe that the future of medicine is federated. Our platform provides the Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) needed to manage these complex linkages at scale. Whether you are looking to accelerate cohort recruitment by 60% or generate regulatory-grade RWE for a new therapy, our Real-time Evidence & Analytics Layer (R.E.A.L.) ensures that your clinical trial data and EHRs aren’t just “talking”—they’re working together to save lives.
By making your trial data and EHRs “best friends,” you aren’t just improving a process; you are building the foundation of a Learning Health System that will drive medical innovation for generations to come.

