Clinical data governance: Secure & Easy 2025
Why Clinical Data Governance is the Foundation of Modern Healthcare Success
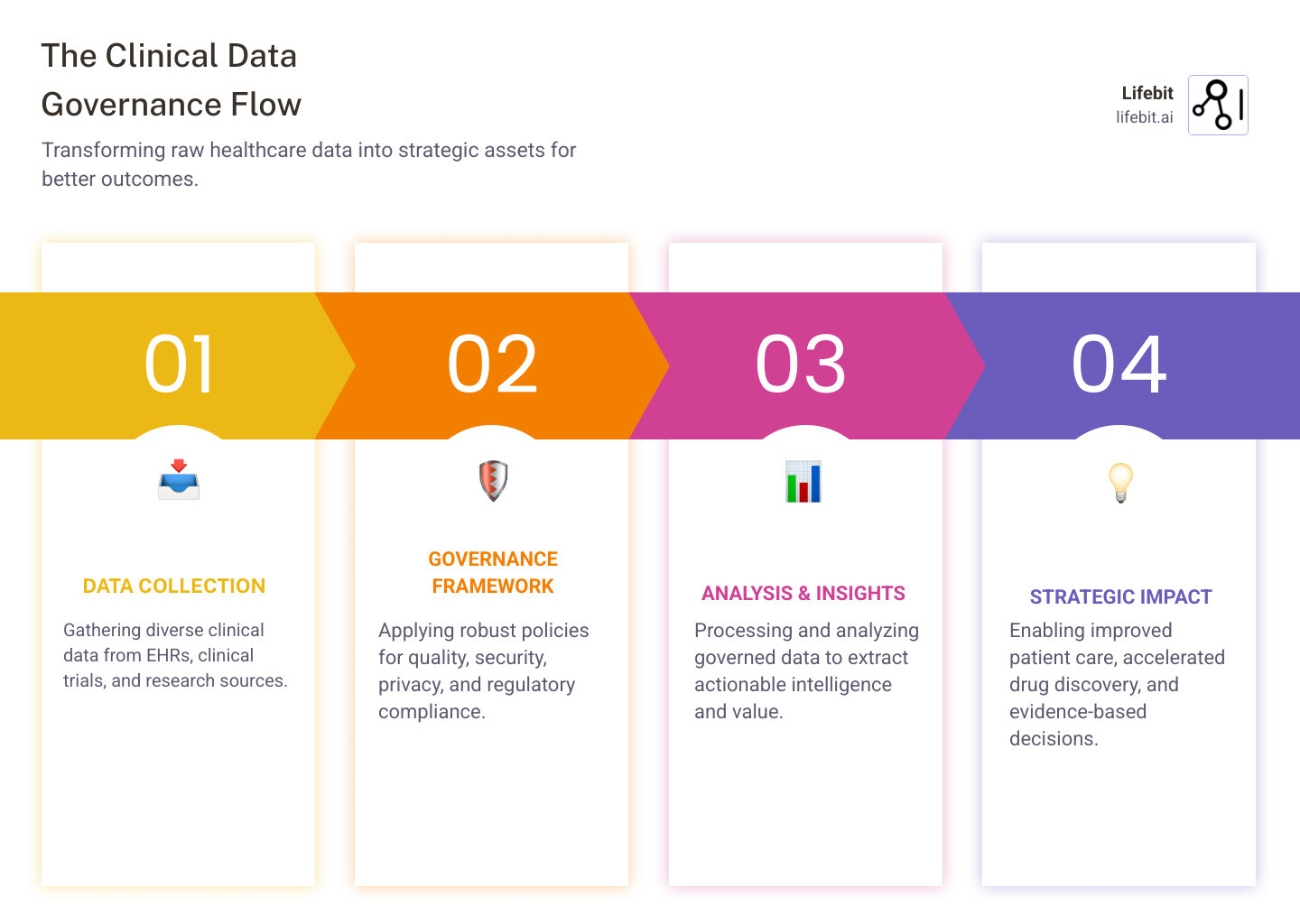
Clinical data governance is the framework for managing healthcare data throughout its lifecycle, ensuring your organization’s most valuable asset—patient data—remains accurate, secure, and compliant. It enables breakthrough findies and better patient outcomes.
What Clinical Data Governance Includes:
- Data Quality Management – Ensuring accuracy, completeness, and consistency
- Security & Privacy Controls – Protecting sensitive patient information
- Regulatory Compliance – Meeting HIPAA, GDPR, and other mandates
- Access Management – Controlling who can see and use data
- Data Lifecycle Oversight – Managing data from creation to archival
- Stakeholder Accountability – Defining clear roles and responsibilities
The stakes are high. Healthcare generates 30% of the world’s data, with hospitals producing 50 petabytes annually. Yet, 68% of healthcare organizations lack a strategy for analytics and AI innovation.
Poor governance has led to over 319,816 HIPAA complaints and $134+ million in penalties since 2003. More critically, inaccurate data can cause misdiagnoses and compromise patient safety.
Effective governance, however, transforms organizations by enabling faster clinical decisions, accelerating drug findy, reducing costs, and building a foundation for AI in healthcare.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology and health-tech, I’ve seen how robust clinical data governance open ups the full potential of healthcare data while upholding the highest standards of privacy and compliance.
Why Clinical Data Governance is Non-Negotiable in Modern Healthcare
Data is the lifeblood of modern healthcare. Every patient visit, lab test, and clinical trial generates a torrent of information that holds the potential to save lives, accelerate research, and optimize operations. However, this data is often fragmented, unstructured, and difficult to harness. The World Economic Forum estimates that the average hospital produces at least 50 petabytes of data annually, with a staggering 80% of it being unstructured in formats like clinical notes, imaging reports, and pathology slides.
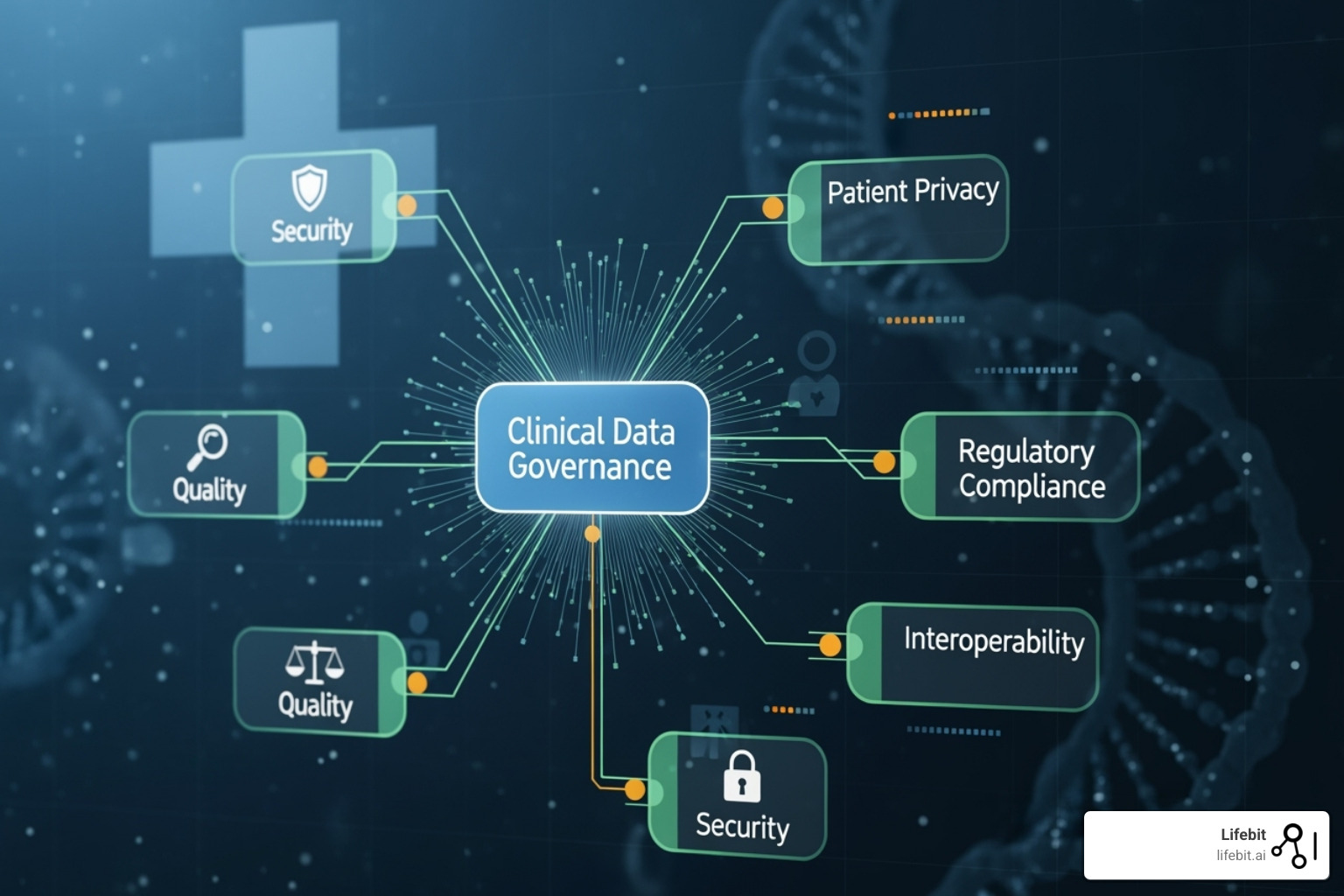
Clinical data governance is the strategic imperative that brings order to this chaos. It establishes the framework of policies, standards, and controls needed to manage data as a valuable enterprise asset, ensuring it is accurate, accessible, secure, and fit for purpose. It is non-negotiable for several critical reasons:
- Patient Safety: In high-stakes clinical environments, decisions are made in seconds based on available information. Accurate, complete, and timely data is paramount. A missing allergy in a patient’s record or an outdated medication list can have catastrophic consequences. Governance ensures that the data clinicians rely on is trustworthy, reducing the risk of preventable medical errors.
- Data-Driven Decisions: The quality of every decision—from an individual patient’s treatment plan to a health system’s multi-million dollar operational strategy—is directly dependent on the quality of the underlying data. Robust governance provides the reliable data foundation needed for confident, evidence-based decision-making at every level of the organization.
- Research and Innovation: Breakthroughs in medicine, from large-scale clinical trials and drug development to the creation of sophisticated AI-powered diagnostic tools, are impossible without high-quality data. Researchers need access to data that is consistent, well-documented, and standardized to produce valid and reproducible results.
- Operational Efficiency: Poor data management creates immense operational drag. Good governance eliminates data silos, reduces redundant data entry, minimizes time spent searching for information, and prevents costly mistakes. This frees up valuable clinical and administrative resources to focus on patient care and strategic initiatives, and it simplifies complex tasks like Preserving Patient Data Privacy and Security.
The High Stakes: Risks of Poor Governance
Neglecting clinical data governance is not a passive choice; it is an active acceptance of devastating, real-world consequences that extend far beyond the IT department.

- Patient Safety Risks: This is the most critical risk. When data is incomplete, inaccurate, or scattered across disconnected systems, providers are forced to make decisions without a complete clinical picture. A study by Johns Hopkins suggests that medical errors are the third-leading cause of death in the U.S., and many of these errors are rooted in poor data and communication.
- Data Breaches and Security Failures: The healthcare industry is a prime target for cyberattacks. Poor governance, characterized by weak access controls and inconsistent security protocols, makes sensitive patient data highly vulnerable. The consequences include not only the loss of data but also the erosion of patient trust, which can take years to rebuild.
- Crippling Regulatory Penalties: Non-compliance with regulations like HIPAA and GDPR is not taken lightly. Since April 2003, the Office for Civil Rights (OCR) has received over 319,816 HIPAA complaints, leading to financial settlements and civil money penalties totaling hundreds of millions of dollars. These fines can cripple an organization’s budget.
- Spiraling Operational Costs: In a poorly governed environment, staff waste countless hours on low-value tasks: manually correcting data entry errors, cross-referencing information between systems, and chasing down missing data. This operational friction translates directly into higher labor costs and lower productivity.
- Stifled Innovation: Without a foundation of trusted data, advanced analytics and AI initiatives are doomed to fail. Organizations find themselves unable to leverage their data for predictive modeling, personalized medicine, or population health management, causing them to fall behind more data-mature competitors.
- Reputational Damage: A major data breach or a high-profile incident related to poor data quality can cause irreparable harm to an organization’s reputation. Patients may choose to seek care elsewhere, and research partners may become hesitant to collaborate, undermining the organization’s mission and financial stability.
The Rewards: Benefits of a Robust Framework
Conversely, organizations that invest in a robust clinical data governance framework open up changeal benefits that create a powerful competitive advantage:
- Improved Clinical Outcomes: With access to a complete, accurate, and longitudinal view of each patient, clinicians can make better, faster decisions. This leads to more accurate diagnoses, personalized treatment plans, fewer medical errors, and ultimately, better patient outcomes.
- Significant Cost Reduction: Efficient data processes lead to direct and indirect savings. Automating data quality checks reduces the need for manual remediation. Eliminating duplicate lab tests and procedures saves money. Streamlined reporting and analytics processes free up expensive data science resources for higher-value work.
- Improved Strategic Decision-Making: Reliable data empowers leaders to make confident, evidence-based choices. From optimizing hospital bed allocation and managing supply chains to identifying opportunities for service line expansion, trustworthy data is the bedrock of sound strategy.
- Accelerated Research and Innovation: High-quality, well-governed data is the fuel for groundbreaking research. It enables the development of precision medicine protocols, accelerates drug findy timelines, and supports the creation of AI/ML models that can predict disease risk or identify novel therapeutic targets.
- Increased Stakeholder Trust: A demonstrable commitment to protecting patient data and ensuring its quality builds deep trust with patients, providers, researchers, and regulatory bodies. This trust is a valuable asset that strengthens relationships and creates a competitive edge.
- A Solid Foundation for AI/ML: A mature governance framework is a prerequisite for successfully implementing AI and machine learning technologies. AI models are only as good as the data they are trained on; governance ensures that this data is clean, consistent, well-documented, and ready for advanced analysis.
The choice isn’t whether to implement clinical data governance—it’s whether to do it proactively to drive strategic advantage, or reactively after suffering the severe consequences of poor data management.
The Core Components of an Effective Clinical Data Governance Framework
An effective clinical data governance framework is not a single piece of software or a static policy document. It is a dynamic, living system that integrates three essential pillars: people, policies, and technology. When orchestrated correctly, these components work in harmony to transform data from a potential liability into a powerful strategic asset.
Think of governance as the operating system for your organization’s data, providing the structure and rules that ensure information flows smoothly, securely, and meaningfully. The key foundational elements of this system include:
- Executive Sponsorship: Lasting change requires top-down support. When executive leaders champion data governance, they signal its importance, secure necessary resources, and ensure that governance objectives are tightly aligned with broader organizational goals.
- Data Stewardship: This is the operational backbone of governance. It involves assigning formal responsibility for specific data assets to individuals or teams who have the subject matter expertise to manage them effectively, ensuring data quality and fitness for purpose.
- Data Lifecycle Management: This is a comprehensive approach that governs how data is handled from its initial creation or acquisition through its active use, sharing, archival, and eventual destruction. A well-defined lifecycle ensures data integrity, security, and compliance at every stage.
- Data Standardization: Healthcare data comes from countless sources—EHRs, labs, imaging systems, wearables, and more. To break down data silos and enable interoperability, organizations must adopt and enforce common data standards (e.g., HL7, FHIR, SNOMED CT, LOINC) and terminologies. Addressing Health Data Standardisation: Technical Challenges is a critical step toward creating a consistent, unified data landscape.
People: Establishing Clear Roles and Responsibilities
Technology and policies are inert without people to bring them to life. Effective governance depends on a well-defined structure of roles and responsibilities, ensuring clear accountability for the organization’s data assets. Key roles include:
- Data Governance Council (or Committee): This is the central governing body, a cross-functional team composed of senior leaders from clinical, research, IT, legal, compliance, and business units. The council is responsible for setting enterprise-wide data policies, prioritizing governance initiatives, resolving data-related issues, and monitoring the program’s progress.
- Chief Data Officer (CDO) or Executive Sponsor: This senior executive is the ultimate champion for data governance. The CDO’s role is to articulate the vision for data as a strategic asset, align governance efforts with business objectives, secure funding and resources, and report on the program’s value to the board and executive team.
- Data Owners: These are typically senior business or clinical leaders who have ultimate accountability for specific data domains (e.g., the Chief Medical Officer might own patient clinical data). They are responsible for approving data policies and access rules for their domain and making high-level decisions about its appropriate use.
- Data Stewards: These are the hands-on guardians of the data. They are subject matter experts, often embedded within business or clinical units, who are responsible for the day-to-day management of data quality. Their tasks include defining data elements, documenting business rules, monitoring data quality metrics, and investigating and resolving data issues.
- Data Custodians: This role is typically held by the IT department. Data custodians are responsible for the technical implementation and management of the data infrastructure. They ensure that data is stored securely, backed up properly, and that the technical systems enforce the access and security policies defined by data owners and the governance council.
Fostering a data-driven culture is the secret ingredient that binds these roles together. This involves moving beyond formal roles to instill a shared sense of responsibility for data quality across the entire organization. Through continuous training, clear communication, and performance incentives, everyone—from the registration clerk entering patient demographics to the researcher analyzing trial results—comes to understand their vital role in maintaining the integrity of this shared asset.
Policies & Processes: The Rulebook for Your Data
Policies and processes are the rulebook that translates the goals of governance into consistent, repeatable actions. They provide clear guidance on how data should be managed, used, and protected. As the management consulting firm Gartner notes, effective governance means “[ensuring] the appropriate behavior in the valuation, creation, consumption and control of data and analytics.”
Essential policies and processes include:
- Data Quality Standards: This goes beyond vague notions of “good data.” It involves defining clear, measurable standards across multiple dimensions: Accuracy (data reflects the real world), Completeness (no missing values), Consistency (data is not contradictory), Timeliness (data is up-to-date), Validity (data conforms to a defined format), and Uniqueness (no duplicate records). These standards must be supported by processes for data profiling, validation, cleansing, and continuous monitoring.
- Access Controls and Management: These policies define who can access what data, under what circumstances, and for what purpose. Modern frameworks use a principle of least privilege, often implemented through Role-Based Access Control (RBAC) or more granular Attribute-Based Access Control (ABAC). These policies must be supported by robust processes for requesting, approving, and auditing data access.
- Data Security Protocols: This encompasses a comprehensive set of technical and administrative measures to protect data from unauthorized access or breaches. It includes policies on data encryption (at rest and in transit), network security, vulnerability management, and a detailed incident response plan.
- Data Sharing and Interoperability Agreements: These policies govern how data is shared securely with external partners, such as other healthcare providers, research institutions, or public health agencies. They define the legal, technical, and ethical requirements for data exchange.
- Data Retention and Archival: Not all data needs to be kept forever. These policies define how long different types of data should be retained and how they should be securely archived or destroyed, based on clinical utility, research value, and legal or regulatory requirements.
Best Practices for Implementing Clinical Data Governance
Launching a successful governance program requires a pragmatic, iterative approach:
- Start small and demonstrate value: Don’t try to boil the ocean. Begin with a pilot project focused on a high-impact area, such as improving the quality of patient demographic data to reduce billing errors or standardizing medication data to improve reconciliation. A quick win builds momentum and demonstrates the tangible value of governance to stakeholders.
- Align with organizational priorities: Frame governance not as a compliance exercise but as an enabler of key business goals. Connect your efforts directly to strategic objectives like reducing hospital readmission rates, accelerating clinical trial enrollment, or improving patient satisfaction scores.
- Ensure clear accountability: Ambiguity is the enemy of governance. Clearly define and communicate the roles and responsibilities of owners, stewards, and custodians to ensure everyone understands their part and can be held accountable.
- Foster cross-functional collaboration: Break down organizational silos. The Data Governance Council should be a forum for open dialogue and joint problem-solving, creating solutions that work for the entire organization, not just one department.
- Accept continuous improvement: Data governance is not a one-time project; it’s an ongoing journey. Regularly assess the effectiveness of your policies and processes, solicit feedback from data users, and adapt your framework to meet new challenges and opportunities.
Navigating the Regulatory Maze: HIPAA, GDPR, and Beyond
Healthcare is one of the world’s most regulated industries, and for good reason. The sensitive nature of personal health information demands a robust legal and ethical framework to protect individuals’ privacy and ensure their data is used responsibly. For clinical data governance, these regulations are not burdensome roadblocks; they are essential guardrails that build patient trust and provide a blueprint for ethical data stewardship.

This regulatory environment is dynamic and complex, with new laws and interpretations emerging constantly. A “set it and forget it” approach to compliance is a recipe for disaster. Instead, governance strategies must be proactive, adaptable, and deeply integrated into the fabric of the organization’s data operations.
Understanding Key Compliance Mandates
While specific requirements vary, major global regulations share the common goals of protecting patient privacy, ensuring data security, and enabling the appropriate and beneficial uses of health data.
-
HIPAA (Health Insurance Portability and Accountability Act): The cornerstone of U.S. health data protection, HIPAA’s rules are foundational for any organization handling American patient data.
- The Privacy Rule establishes national standards for the protection of Protected Health Information (PHI). It defines how PHI can be used and disclosed, and it grants patients fundamental rights, including the right to access, amend, and receive an accounting of disclosures of their health information.
- The Security Rule complements the Privacy Rule by setting standards for securing electronic PHI (e-PHI). It mandates specific administrative (e.g., security training, risk analysis), physical (e.g., facility access controls, workstation security), and technical (e.g., access control, encryption, audit logs) safeguards.
- The HITECH Act significantly strengthened HIPAA’s enforcement by increasing penalties for non-compliance, introducing a mandatory breach notification rule, and promoting the adoption of certified EHR technology.
-
GDPR (General Data Protection Regulation): A landmark regulation that has set a global standard for data privacy, the GDPR applies to the processing of personal data of any EU resident. Its core principles are highly influential:
- Key Principles: GDPR mandates that data processing be lawful, fair, and transparent. It requires purpose limitation (data collected for one purpose cannot be used for another without consent), data minimization (collecting only necessary data), and storage limitation (not keeping data longer than necessary). It also demands accuracy, integrity, and confidentiality.
- Individual Rights: It grants extensive rights to individuals, including the right to data portability and the “right to be forgotten” (erasure). You can find more info about GDPR Compliant Data on our blog.
-
21st Century CURES Act: This U.S. law is designed to accelerate medical innovation and promote data interoperability. A key provision is its rule against “information blocking”—the unreasonable interference with the access, exchange, or use of electronic health information. This act pushes healthcare organizations to share data more freely and securely to improve patient care, making robust governance essential to manage this flow.
-
ICH E6(R3) Good Clinical Practice (GCP): These international guidelines are the gold standard for conducting clinical trials. The latest revisions place a strong emphasis on data governance, requiring sponsors to implement systems that ensure the reliability, quality, and integrity of trial data from start to finish.
-
State-Level Regulations (e.g., CCPA/CPRA): The regulatory landscape is further complicated by state-specific laws like the California Consumer Privacy Act (CCPA) and its successor, the California Privacy Rights Act (CPRA). These laws grant consumers, including patients, rights over their personal information that can sometimes extend beyond HIPAA’s protections, requiring organizations to manage data according to multiple legal frameworks.
How Regulations Shape Your Governance Strategy
These complex regulations are not just a checklist for the legal department; they fundamentally shape the design of a practical clinical data governance framework.
- Privacy by Design and by Default: Modern regulations demand that privacy is not an afterthought. Governance frameworks must embed privacy controls—such as data minimization, pseudonymization, and de-identification techniques—into the design of systems and processes from the very beginning.
- Comprehensive Security Controls: Compliance requires a multi-layered security posture. Governance policies must mandate not just basic password protection but a full suite of controls, including end-to-end encryption, multi-factor authentication, continuous vulnerability scanning, and detailed, immutable audit trails for all data access.
- Robust Patient Access Rights Management: A governance framework must include clear processes and technical capabilities to honor patients’ legal rights to access, review, correct, and understand how their health data is being used.
- Secure Interoperability and Vendor Management: Governance must provide a clear framework for balancing the CURES Act’s mandate for data sharing with the privacy risks involved. This includes establishing clear policies, technical standards, and robust legal agreements (like Business Associate Agreements under HIPAA) for any third-party vendor or partner that handles patient data.
- Transparency and Demonstrable Accountability: Organizations must be able to demonstrate compliance. This requires meticulous documentation of data flows, processing activities, risk assessments, and policy decisions, creating an evidentiary trail for regulators and patients.
- Strict and Practiced Breach Notification Rules: With tight reporting deadlines (e.g., 72 hours for some GDPR breaches, 60 days for HIPAA), a governance program must include a well-defined and regularly tested incident response plan to ensure swift and compliant action in the event of a breach.
By embedding these regulatory requirements directly into your governance framework, compliance ceases to be a separate, burdensome activity and becomes a natural outcome of sound, ethical data management.
Technology That Powers Modern Clinical Data Governance
While people and policies form the strategic foundation of clinical data governance, technology is the engine that brings the framework to life. The right technology stack automates enforcement, scales efforts across the enterprise, and transforms governance from a manual, compliance-driven burden into a dynamic, value-creating advantage.
Effective governance in the modern healthcare ecosystem is impossible at scale without a suite of enabling technologies. Key platforms and tools include:
- Data Catalogs: A data catalog acts as a centralized, intelligent inventory of all an organization’s data assets. It automatically scans data sources, extracts metadata (information about the data), and creates a searchable, user-friendly interface. For clinicians and researchers, it’s like a “Google for data,” allowing them to quickly find relevant datasets, understand their lineage, view quality scores, and learn the rules for their use. This dramatically reduces the time spent searching for data and increases trust in its content.
- Master Data Management (MDM) Systems: Healthcare organizations are plagued by duplicate and conflicting records, especially for patients and providers. MDM systems solve this by creating a single, authoritative, trusted version of critical data entities—often called a “golden record.” By linking all instances of a patient’s data from different systems (EHR, billing, lab), MDM ensures a complete, 360-degree view, which is essential for patient safety, care coordination, and accurate analytics.
- Data Lineage Tools: These tools provide a visual map of data’s journey from its source through all changes and systems to its final destination. When a clinician questions a value in a report or a researcher finds an anomaly, data lineage tools allow them to perform root cause analysis, tracing the data back to its origin to identify errors or understand its context. This is crucial for accountability, troubleshooting, and regulatory audits.
- Data Quality Software: Manually finding and fixing data errors across petabytes of information is an impossible task. Automated data quality tools profile datasets to find inconsistencies, validate data against predefined rules, cleanse and standardize information, and monitor quality over time. They provide dashboards that help data stewards proactively identify and remediate issues before they impact downstream processes.
- Access Management and Security Platforms: These are the systems that enforce the rules defined in governance policies. They manage user identities, authenticate users (often with multi-factor authentication), and enforce role-based or attribute-based access controls to ensure users only see the data they are authorized to see. They also provide detailed, tamper-proof audit trails of every access request and action.
Furthermore, artificial intelligence is revolutionizing data governance itself. AI-Enabled Data Governance platforms can boost these efforts by automatically classifying sensitive data, detecting anomalous access patterns that could signal a threat, suggesting new data quality rules based on observed patterns, and proactively identifying potential compliance risks.
The Future is Federated: Secure, Compliant Data Access at Scale
For decades, large-scale data collaboration in healthcare has been built on a broken model: centralizing massive, sensitive datasets into a single location. This approach creates enormous security risks, compliance headaches (especially with data residency laws like GDPR), and logistical nightmares. The future of collaborative research and analytics lies in a fundamentally different, more secure paradigm: a federated approach, where analysis is brought to the data, not the other way around.
Federated Data Governance is a transformative model that allows sensitive patient data to remain securely within its original, trusted institutional boundary. Instead of moving data, researchers or analysts send their computational queries or machine learning algorithms to the data. The analysis is executed locally within the data’s protected environment, and only the aggregated, non-identifiable results are returned to the researcher. The raw patient data never leaves its Secure Data Environment (SDE), enabling groundbreaking research without compromising patient privacy or data security.
This approach open ups previously impossible multi-party collaboration. For example, a pharmaceutical company could train a predictive AI model on cancer imaging data from ten different hospitals across the globe without any of the hospitals ever having to share their raw patient images. This is particularly powerful for real-world evidence studies and research on rare diseases, where combining data from multiple sources is essential to achieve statistical power. Federated governance eliminates the months or even years often spent on complex data use agreements and transfers.
From a security and compliance perspective, the federated model is a game-changer. It inherently solves data residency challenges because the data never crosses borders. It minimizes the attack surface because sensitive data is not aggregated in a single, high-value target. It simplifies compliance with regulations like HIPAA and GDPR because the data controller never loses custody of their data. Our platform at Lifebit demonstrates this in practice, enabling secure, global-scale collaboration that adheres to the highest standards of data protection.
Federated technologies are no longer theoretical; they are being deployed by leading biomedical research and healthcare organizations today. They represent a paradigm shift in how we approach clinical data governance for a collaborative world. The question is no longer if this shift will happen, but how quickly your organization will adopt it to gain a competitive and scientific edge.
Frequently Asked Questions about Clinical Data Governance
Here are the answers to the most common questions about clinical data governance.
What is the difference between data governance and data management?
These terms are often confused, but the distinction is critical.
- Data governance is the blueprint. It’s the strategic framework that defines the rules, policies, and responsibilities for data. It answers the “what” and “why.”
- Data management is the construction. It’s the hands-on implementation of the governance plan—the day-to-day work of collecting, storing, and securing data.
In short, governance is the strategy, and management is the execution. You need both to succeed. It’s important to distinguish between data governance and data management as they are two sides of the same coin.
Who is responsible for clinical data governance in a healthcare organization?
Clinical data governance is a team sport with shared responsibility across the organization:
- Executive Leadership: Champions the initiative, provides resources, and sets the tone from the top.
- Data Governance Council: A cross-functional team (clinical, IT, legal, business) that sets policies and oversees the program.
- Data Owners: Business leaders accountable for specific data assets (e.g., patient records, billing data).
- Data Stewards: Frontline staff who work with the data daily and are responsible for its quality.
- IT Department: Acts as the data custodian, managing the technical infrastructure and security.
- Clinical Staff: Play a crucial role in ensuring data accuracy at the point of care.
- Compliance Officers: Guide the organization through the complex regulatory landscape.
The key is a culture where everyone understands data is a vital, shared asset.
How do you measure the success of a data governance program?
You can’t manage what you don’t measure. Success in clinical data governance can be tracked with key metrics:
- Data Quality Improvements: Track reductions in data entry errors, higher field completion rates, and improved data consistency.
- Compliance and Risk Reduction: Measure decreases in data breaches, fewer audit findings, and faster resolution of compliance issues.
- Operational Efficiency Gains: Monitor time saved on data cleanup, reduced time-to-insight for analytics, and cost savings from streamlined processes.
- User Satisfaction: Gauge whether clinical teams trust the data more and are adopting new policies, using surveys and feedback.
- Impact on Innovation: Assess the ability to make faster, more confident decisions and launch more data-driven initiatives.
Measuring these outcomes demonstrates value and creates a virtuous cycle of continuous improvement.
Conclusion: Building a Future-Proof, Data-Driven Organization
Clinical data governance is no longer just about managing data; it’s about building the foundation for the future of healthcare. While organizations are drowning in data, the key to thriving is governing it well.
Effective governance improves patient safety, reduces costs, and accelerates medical findies. It also provides the framework to confidently steer the complex regulatory landscape of HIPAA, GDPR, and beyond, avoiding costly penalties and reputational damage.
The future is federated, moving away from risky data transfers to a model where analysis happens securely where the data lives. This approach open ups unprecedented collaboration while upholding the highest privacy standards.
Clinical data governance is not a one-time project but a continuous journey of strategic investment. The framework you build today is essential for tomorrow’s innovations, from AI-powered diagnostics to accelerated clinical trials. It all starts with governed, trustworthy data.
Ready to build a future-proof, data-driven organization? Find how to accelerate your research with a modern approach to data governance and see how Lifebit’s federated platform can help.

