Clinical Variant Interpretation Made Easy—Your Genomics GPS

Clinical Variant Interpretation: 5 Easy Tiers
Decoding Your DNA Blueprint
Clinical variant interpretation is the process scientists and doctors use to determine what changes in your DNA mean for your health. It’s the crucial step that translates raw genetic data into actionable medical insights.
In short, clinical variant interpretation involves:
- Identifying DNA changes: Finding variations in genes compared to a standard reference.
- Assessing significance: Determining if these changes are harmless, cause disease, or have an unknown effect.
- Guiding medical care: Using this information for diagnosis, treatment, and understanding disease risk.
Modern technologies like Next-Generation Sequencing (NGS) allow us to read more of our DNA than ever, which is a major advance for diagnosing genetic disorders. However, this also means we find a vast number of DNA changes, many of which have never been seen before. Figuring out their meaning is a significant challenge.
This guide will help you understand this complex process, showing you how geneticists interpret these variants and how you can better understand genetic reports.
I’m Dr. Maria Chatzou Dunford, and my work at Lifebit focuses on making complex genomic data accessible for clinical variant interpretation. My background in computational biology and AI has been dedicated to building tools that empower precision medicine.
The Foundation: Understanding Variant Classification with ACMG/AMP Guidelines
To ensure consistency in clinical variant interpretation, labs worldwide follow a roadmap created by the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP). These landmark 2015 guidelines provide a standardized, evidence-based framework to ensure a variant is interpreted the same way, regardless of where the test is performed. This consistency is critical for accurate diagnosis, risk assessment, and treatment decisions.
The Five Tiers of Variant Significance
The ACMG/AMP system classifies each variant into one of five categories based on the strength and nature of the available evidence.
- Pathogenic (P): Reserved for variants with >99% certainty of causing disease. The evidence is robust and unequivocal. These changes are known to directly lead to specific genetic conditions. For example, the ΔF508 variant in the CFTR gene is a well-established pathogenic cause of Cystic Fibrosis.
- Likely Pathogenic (LP): Assigned to variants with a high likelihood (~90-99% certainty) of causing disease. The evidence is strong, but may fall just short of the definitive proof required for a pathogenic classification. These variants are considered to have clinical actionability, meaning clinicians can confidently use this information to guide medical management.
- Variant of Uncertain Significance (VUS): This category is for variants where the evidence is insufficient, weak, or conflicting. We cannot confidently determine if they are harmful or harmless. A VUS is not a statement about risk; it is a statement about the current limits of our knowledge.
- Likely Benign (LB): Variants with a high likelihood (~90-99% confidence) that they do not cause disease. The evidence suggests they are harmless, but there might be a small, residual uncertainty.
- Benign (B): Variants that are definitively harmless, with >99% confidence. They are considered normal variations in the human genome, often found at a significant frequency (typically >5% of the population) in large-scale population databases.
You can dive deeper into these classifications by checking out the original Standards and guidelines for sequence variant interpretation.
Types of Evidence in Variant Curation
To classify a variant, scientists gather evidence using specific evidence codes (e.g., PVS1, PM2, PP3, BS1) from the ACMG/AMP framework. Each code has a defined strength (Very Strong, Strong, Moderate, or Supporting) and is combined with other codes to reach a final classification.
- Population data: How common is a variant in the general population? Databases like gnomAD are used to check frequencies. A variant that is too common for a rare disease is evidence for being benign (BS1), while being absent from controls is moderate evidence for pathogenicity (PM2).
- Computational data: Software tools like PolyPhen-2, SIFT, and CADD predict whether a genetic change will damage protein function. These predictions provide supporting evidence (PP3 for pathogenic, BP4 for benign) but require confirmation from other evidence types.
- Functional data: Laboratory experiments test what a variant actually does to a gene or protein. A well-established study showing a damaging effect provides strong evidence for pathogenicity (PS3), while a study showing no effect is strong evidence for it being benign (BS3).
- Segregation data: This involves looking at families to see if a variant tracks with a disease across generations. Co-segregation with disease in multiple family members is supporting evidence for pathogenicity (PP1).
- De Novo Data: A variant confirmed to have occurred for the first time in a patient (and is absent in their parents) is strong evidence for pathogenicity (PS2), assuming a good gene-phenotype match.
- Variant Type: A “null” variant (e.g., nonsense, frameshift) in a gene where loss-of-function is a known disease mechanism is considered very strong evidence for pathogenicity (PVS1).
The framework is not static. Groups like the ClinGen Sequence Variant Interpretation Working Group continually refine these guidelines, with expert panels developing gene-specific rules to reduce subjectivity.
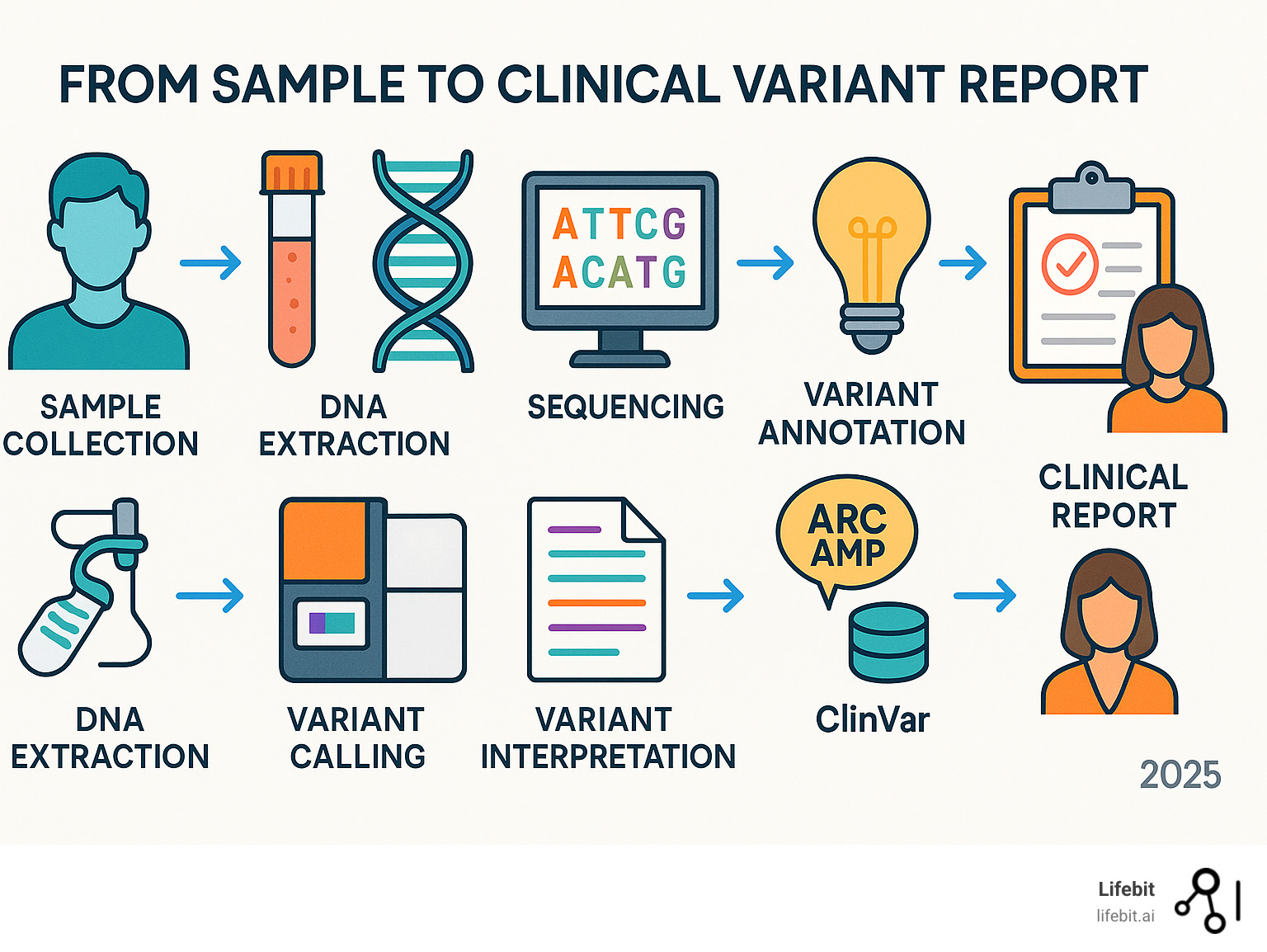
A Step-by-Step Guide to Clinical Variant Interpretation

The process of clinical variant interpretation follows a logical sequence, where each step builds on the last to create a complete picture for patient care.
Step 1: Gather Variant and Gene-Specific Information
Before interpretation, we must understand the context. This involves examining the gene-disease relationship: is the gene robustly associated with disease? What is the inheritance pattern (e.g., autosomal dominant or recessive)? We also identify the variant type, as this provides initial clues about its potential impact. Key types include:
- Nonsense, Frameshift, and Canonical Splice Site Variants: Often lead to a loss of function and are strong indicators of pathogenicity if that is the known disease mechanism.
- Missense Variants: Swap one amino acid for another, with highly variable effects that require further investigation.
- Copy Number Variants (CNVs): Deletions or duplications of large DNA segments.
A nonsense variant in a gene where function loss causes disease is strong evidence for pathogenicity. We check allele frequency databases like gnomAD to see how common the variant is; a high frequency in healthy populations suggests it is benign. Finally, we ensure the analysis uses the correct reference transcript (e.g., MANE Select) to represent the most clinically relevant version of the gene. For more background, see A guide to genetic testing.
Step 2: Apply Evidence Criteria Using the ACMG/AMP Framework
This step involves systematically applying the ACMG/AMP evidence criteria. For example, a novel missense variant in a cancer-risk gene might be evaluated as follows:
- Population data: It is absent in gnomAD (PM2 – Moderate).
- Computational predictors: Tools like PolyPhen-2 and SIFT predict a damaging effect (PP3 – Supporting).
- Segregation data: It is observed to co-segregate with cancer in the patient’s family (PP1 – Supporting).
- Functional studies: No published studies exist.
By combining these codes (e.g., 1 Moderate + 2 Supporting), the evidence may be sufficient to classify the variant as Likely Pathogenic, a clinically actionable result.
The ClinGen Sequence Variant Interpretation Working Group provides refined guidance for applying these criteria in complex scenarios, such as for variants affecting RNA splicing.
Step 3: Use Key Databases and Expert Panels
Modern clinical variant interpretation relies on shared knowledge. The ClinVar database is a primary resource, containing variant interpretations from labs worldwide. Its review status (star rating) helps users gauge the confidence level, from single submissions (one star) to classifications from expert panels (four stars). While interpretations can differ, The ClinVar database for variant assertions provides crucial context and supporting evidence. The Human Gene Mutation Database (HGMD) also catalogs disease-causing mutations from the literature.
Disease-specific expert panels, often organized by ClinGen, develop customized guidelines for interpreting variants in specific genes like PTEN or TP53. This collaborative approach, built on data sharing, improves accuracy and consistency globally.
Step 4: Synthesize Evidence and Assign a Final Classification
The final step is to weigh all the evidence, including any conflicting points, to assign a classification. This requires scientific rigor and expert judgment. Labs must carefully document the rationale for their decision, creating a transparent record that allows for future re-evaluation. The variant is assigned one of the five ACMG/AMP categories: Pathogenic, Likely Pathogenic, VUS, Likely Benign, or Benign.
Because genetics is a rapidly evolving field, classifications must be re-evaluated over time. A VUS today may be reclassified as new research becomes available. Laboratories should have protocols for periodically reviewing variants to ensure patients receive care based on the latest scientific understanding.
Navigating Key Challenges in Variant Interpretation

Despite significant advances, clinical variant interpretation faces persistent challenges. The complexity of genetic reports can be difficult for non-specialists to steer, and the sheer volume of data from modern sequencing creates issues for labs and patients alike.
The VUS Dilemma: Managing Uncertainty in Clinical Practice
The Variant of Uncertain Significance (VUS) is one of the biggest challenges. A VUS classification means there is not enough information to know if a genetic change is harmful or benign. This creates significant patient anxiety and leaves them in diagnostic limbo. For clinicians, a VUS is not clinically actionable; it cannot be used to confirm a diagnosis or guide treatment, often leading to a “wait and see” approach.
A critical issue is that VUS rates are significantly higher in underrepresented populations due to the lack of diversity in genetic databases. This disparity creates inequities in the diagnostic power of genetic testing, a major focus for the genomics community to resolve.
Strategies to reduce VUS rates include family studies, functional lab experiments, and new high-throughput technologies like Multiplexed Assays of Variant Effect (MAVEs). These efforts are showing success; for example, 49% of VUS in the BRCA1 gene have been reclassified using systematic evidence integration.
Ensuring Consistency and Quality Over Time
Different labs can sometimes classify the same variant differently. While the ACMG/AMP guidelines provide a framework, some professional judgment is still required, leading to inter-laboratory discrepancies. To combat this, robust labs use detailed standard operating procedures, participate in proficiency testing programs, and rely on data sharing through ClinVar to move toward consensus.
Furthermore, genetic knowledge is constantly evolving. A variant’s classification may change as new research emerges. Therefore, labs must have systems for periodically re-evaluating variants, especially VUS, to ensure interpretations remain current. Quality genetic testing reports are essential, clearly stating the findings, the supporting evidence, and the clinical implications.
Best Practices for Communicating Clinical Variant Interpretation Results
Effective communication is critical. Reports should be clear, avoid jargon, and provide actionable recommendations for clinicians. Patient-friendly summaries are also valuable for translating complex findings into understandable language.
Genetic counselors play a vital role in bridging the gap between the lab and the patient. They are trained to explain complex results, manage uncertainty around a VUS, and provide support. Best practices include discussing the possibility of a VUS result before testing begins to manage patient expectations.
The Future is Now: Advanced Technologies Improving Variant Interpretation
The field of clinical variant interpretation is advancing at a rapid pace, with new technologies leveraging massive datasets and artificial intelligence to provide deeper insights into our DNA.
How AI and Automation are Enhancing Clinical Variant Interpretation
The sheer volume of data from Next-Generation Sequencing (NGS) is too large for manual review. Artificial Intelligence (AI) and machine learning (ML) are changing this process. AI-powered evidence gathering uses Natural Language Processing (NLP) to rapidly sift through millions of scientific papers, while automated classification tools use algorithms trained on previously classified variants to predict whether a new variant is harmful. Advanced deep learning models, such as SpliceAI, can predict complex functional consequences with high accuracy. While human experts provide the final review, these tools offer a powerful first-pass analysis, improving efficiency and consistency.
At Lifebit, our next-generation federated AI platform is built to securely access and analyze global biomedical data. With capabilities for data harmonization and advanced AI/ML analytics, we empower large-scale, compliant research. Our platform uses AI to ensure variant interpretations are continuously updated with the latest scientific evidence, making clinical variant interpretation more efficient, accurate, and scalable. For More info about AI-driven analytics for genomics, visit our solutions page.
The Power of Functional Genomics at Scale
A major advance is the ability to perform laboratory experiments on thousands of variants simultaneously, providing functional evidence at an unprecedented scale.
Multiplexed Assays of Variant Effect (MAVEs) allow scientists to create a library of thousands of variants, introduce them into cells, and measure the functional consequence of each one in a single experiment. Similarly, saturation genome editing using tools like CRISPR can create a complete “look-up table” of what each possible variant in a gene does.
These high-throughput functional studies are critical for solving the VUS dilemma. Resolving VUS with functional data is highly effective; studies have shown that 49% of VUS in BRCA1, 69% in TP53, and 15% in PTEN were reclassified using this type of evidence. This work turns “unknowns” into actionable clinical insights. This approach of proactive variant effect mapping aims to create comprehensive functional datasets for genes before a VUS is even identified in a patient, making future interpretations faster and more accurate.
This can be combined with patient-specific multi-omics data. For instance, transcriptomics (RNA-seq) can confirm if a variant suspected to affect splicing is actually causing an abnormal transcript in the patient’s own cells, providing powerful, personalized functional evidence.
Frequently Asked Questions about Clinical Variant Interpretation
We understand that diving into clinical variant interpretation can bring up many questions. Here are answers to some of the most common queries.
What is the difference between a pathogenic and a likely pathogenic variant?
The difference lies in the level of certainty, as defined by the ACMG/AMP classification system.
- A Pathogenic (P) variant is one we are over 99% certain causes a specific disease. The evidence is overwhelming and has been validated across multiple studies, allowing for clear clinical action. For example, certain variants in the SCN1A gene are known pathogenic causes of Dravet syndrome.
- A Likely Pathogenic (LP) variant is one where we are over 90% certain it causes disease. The evidence is strong, but may lack a piece of definitive data that would lift it to pathogenic. These variants are still considered clinically actionable.
While both classifications indicate a disease-causing variant, the slight difference in certainty can sometimes influence clinical management.
Why do different labs sometimes classify the same variant differently?
This can happen for a few reasons, even with standardized ACMG/AMP guidelines.
First, there is still room for expert judgment in weighing different types of evidence. Second, labs may have access to different internal or private data that is not publicly available. Finally, the field of genomics is constantly evolving. One lab may have incorporated new research that another has not yet reviewed.
This is why public databases like The ClinVar database for variant assertions are so critical. They promote transparency and allow the genetics community to work toward a consensus classification by sharing interpretations and evidence. Open data sharing is key to building a more accurate and consistent knowledge base for everyone.
My report shows a VUS. What happens next?
A Variant of Uncertain Significance (VUS) means there is currently not enough information to determine if the variant is harmful or benign. A VUS does not typically lead to immediate changes in medical care.
The classification is not necessarily permanent. Laboratories should periodically re-evaluate VUS classifications as new research becomes available. A VUS today might be reclassified in the future.
One of the most helpful next steps is often family studies, which involves testing relatives to see if the variant tracks with the disease in the family. This can provide strong evidence to help reclassify the variant. It is important to stay in touch with your healthcare provider or genetic counselor about the status of a VUS.
Conclusion: Empowering Precision Medicine Through Accurate Interpretation
We’ve explored the complex world of clinical variant interpretation, from the foundational ACMG/AMP guidelines to the advanced technologies shaping its future. The process is a form of scientific detective work, piecing together clues from population, computational, functional, and family data to determine a variant’s impact on health. It is a continuous cycle of finding and refinement.
Accurate and consistent clinical variant interpretation is the critical bridge between raw genomic data and actionable clinical insights. It empowers personalized diagnoses, guides targeted treatments, and leads to better patient outcomes. While challenges like the VUS dilemma and inter-laboratory discrepancies remain, they highlight the need for continued standardization and data sharing.
The future is bright, with AI and machine learning accelerating evidence gathering and powerful functional genomics tools systematically resolving VUS at scale. These advancements are making variant classification faster, more precise, and less subjective.
This is where Lifebit steps in. Our next-generation federated AI platform is built to manage this complexity. We provide secure, real-time access to global biomedical data, enabling compliant, large-scale research. With tools for harmonization, advanced AI/ML analytics, and secure collaboration, we help researchers and clinicians steer genomic data to deliver real-time insights for precision medicine.

