Disease Registries 101: Your Essential Guide

Disease Registries 101: Your Essential Guide
Why Disease Registries Are Critical to Modern Healthcare
Disease registries are organized systems for collecting, storing, and analyzing longitudinal health data on patients with specific conditions. Far more than simple databases, they are dynamic, powerful tools that serve multiple, critical purposes across the healthcare ecosystem.
Key Functions in Detail:
- Patient recruitment for clinical trials: Registries create a pre-qualified and engaged pool of potential participants. This dramatically reduces the time and cost of recruitment, one of the most significant bottlenecks in drug development. Researchers can quickly identify individuals who meet specific trial criteria, accelerating the launch of vital studies.
- Natural history tracking: For many conditions, especially rare diseases, the natural progression of the illness is poorly understood. Registries track patients over time to establish a baseline understanding of the disease’s course, identify key milestones, and measure the rate of progression without intervention. This natural history data is indispensable for designing effective clinical trials and serves as a benchmark against which new treatments can be evaluated.
- Quality improvement in healthcare: By collecting data on treatments and outcomes from different providers, registries can identify best practices and highlight variations in care. For example, a registry might reveal that one hospital has significantly better outcomes for a specific surgery. This insight allows other institutions to adopt those successful protocols, leading to system-wide improvements in patient safety and quality of care.
- Real-world evidence (RWE) generation: While randomized controlled trials (RCTs) are the gold standard for proving efficacy, they occur in highly controlled environments with narrow patient populations. Registries collect Real-World Data (RWD) from everyday clinical practice, reflecting a broader, more diverse group of patients with multiple comorbidities. The analysis of this RWD generates RWE, which provides crucial insights into how treatments perform in the real world, informing regulatory decisions and clinical guidelines.
- Population health monitoring and surveillance: Registries are essential for epidemiology. They track the incidence (new cases) and prevalence (total cases) of diseases within a defined population. This data helps public health officials monitor trends, understand risk factors, allocate resources effectively, and plan for future healthcare needs.
Using observational methods, registries gather uniform data for predetermined scientific, clinical, or policy purposes. This systematic approach is fundamentally changing how we understand and treat diseases, a role underscored by guidance from bodies like the Agency for Healthcare Research and Quality (AHRQ).
The impact is significant. There are now over 800 rare disease registries in Europe alone, and major cancer registries like the U.S. SEER Program track outcomes for millions of patients, providing invaluable data for cancer control and research. From the New York State CABG Registry, which has driven major improvements in cardiac surgery outcomes, to patient-powered networks for rare conditions, these systems are reshaping medical research and care. For both common and rare diseases, registries provide the essential foundation for evidence-based medicine and accelerated drug development.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve built computational tools to transform biomedical data analysis, including for disease registries. My work focuses on secure, federated platforms that enable global collaboration while protecting patient privacy—a critical need for modern registry networks.
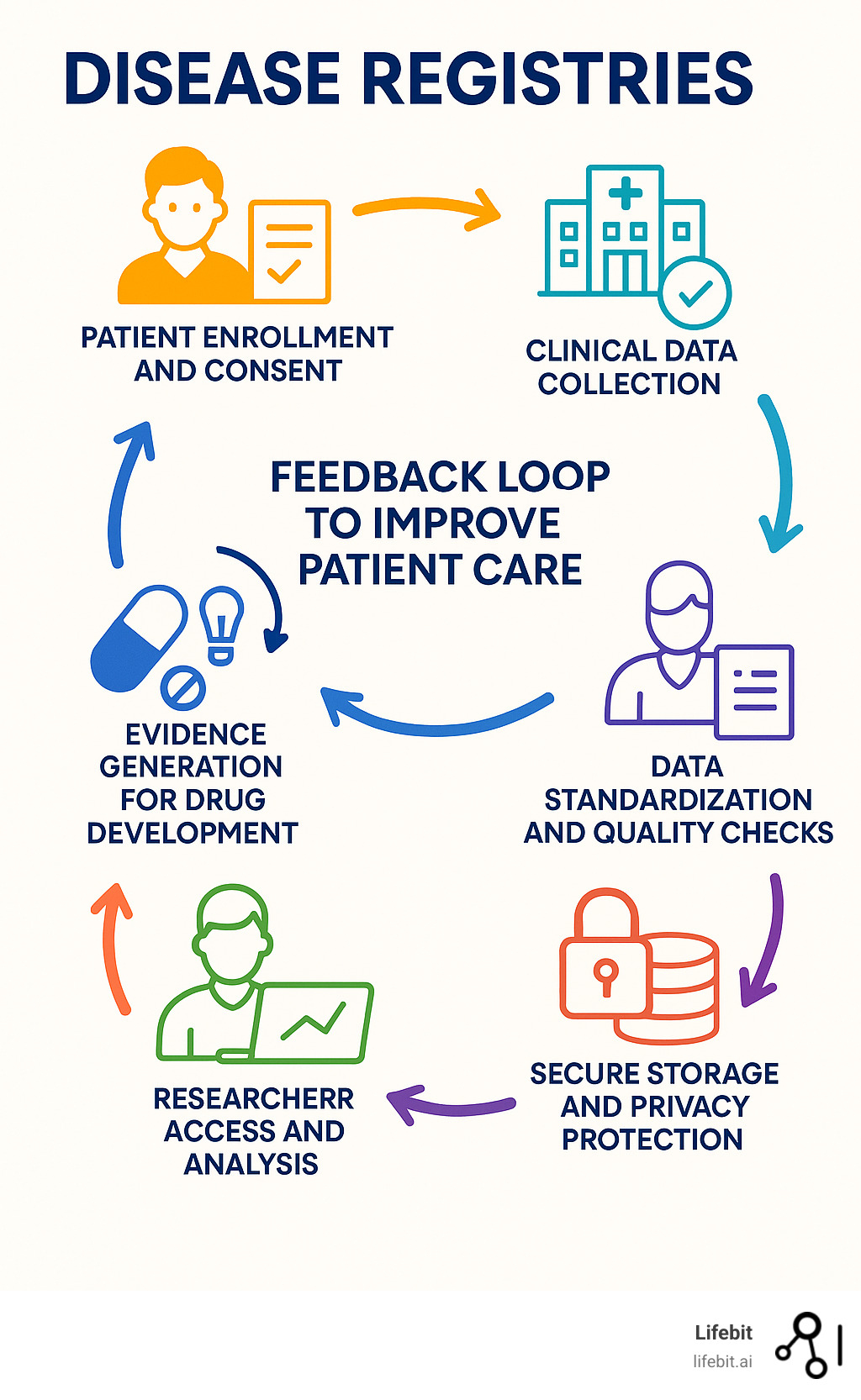
Disease registries terms explained:
The Core Types of Disease Registries
Disease registries use observational study methods, collecting data on events as they happen in routine clinical practice without mandating specific interventions. This approach provides invaluable real-world insights into disease progression, treatment effectiveness, and patient safety. Data sources are diverse, ranging from structured electronic medical records (EMRs) and insurance claims to unstructured clinician notes and direct patient-reported data.
Hospital-Based vs. Population-Based Registries
A fundamental distinction among disease registries is their scope. Hospital-based registries are confined to a single facility or healthcare system. They are powerful tools for internal quality improvement, tracking outcomes for specific procedures, and supporting local research. However, their limited scope means they can suffer from selection bias and may not represent the broader patient community, as they only capture individuals treated at that specific institution.
In contrast, population-based registries aim to capture all cases of a specific disease within a defined geographic area (e.g., a city, state, or country). This makes them crucial for epidemiology, as they can accurately determine disease incidence and prevalence, monitor public health trends, and inform policy. To achieve this comprehensive view, they must collect data from numerous sources, including hospitals, private clinics, pathology labs, and death certificates. Prominent examples include the New York State CABG Registry and the U.S. SEER (Surveillance, Epidemiology, and End Results) cancer registries, which provide vital, generalizable data on a large scale.
Here’s a quick comparison:
| Feature | Hospital-Based Registries | Population-Based Registries |
|---|---|---|
| Scope | Single institution or healthcare system | Defined geographic region (city, state, country) |
| Purpose | Quality improvement, internal research, clinical management | Epidemiology, public health planning, policy, surveillance |
| Data Sources | EMRs, clinician reports, hospital records | EMRs, clinician reports, pathology labs, death certificates, multiple healthcare providers |
| Strengths | Detailed clinical data, easier to establish, good for quality control | Generalizable findings, accurate prevalence/incidence rates, ideal for public health |
| Limitations | Selection bias, not representative of the whole population | More complex and costly to manage, data collection can be less detailed |
| Examples | A hospital’s internal breast cancer program registry | New York State CABG Registry, SEER Cancer Registries |
Product and Health Services Registries
Beyond disease-focused registries, other types serve specific purposes:
- Product Registries: These track outcomes associated with a particular medical product, such as a drug or medical device (e.g., a pacemaker or joint implant). They are primarily used for post-market surveillance to monitor long-term safety and effectiveness, often to fulfill regulatory requirements. They are critical for detecting rare or delayed adverse events that may not appear in pre-market clinical trials.
- Health Services Registries: These focus on outcomes related to specific medical procedures or healthcare services. For example, a registry might track patients undergoing a certain type of bariatric surgery or receiving a specific cancer therapy protocol, regardless of the underlying disease. Their goal is to evaluate the effectiveness, quality, and value of care delivery.
The Rise of Patient-Powered Registries (PPRs)
A powerful alternative to traditional, researcher-led models is the Patient-Powered Registry (PPR). As the name suggests, PPRs are initiated and governed by patients and their families, often through patient advocacy groups (PAGs). In this model, patients are not just subjects but active partners who control their data, help define the research agenda, and participate in disseminating findings.
This deep engagement fosters immense trust and a sense of ownership, which is especially impactful for rare diseases where patients are geographically dispersed and highly motivated. Many PPRs arise from an urgent need within patient communities to accelerate research, changing patients into active citizen scientists. A study found that forty-five percent of disease advocacy organizations supported a research registry or biobank, highlighting their commitment to driving progress.
The success of PPRs hinges on transparency and continuous engagement. When patients understand how their data is being used and see tangible progress, they are more motivated to contribute high-quality, longitudinal data. An excellent Example of a successful patient registry is the Cystic Fibrosis Foundation Patient Registry. For decades, it has tracked the natural history of CF, informed the development of groundbreaking drugs like Trikafta, and dramatically improved standards of care, showcasing the immense value of patient leadership.
How Registries Accelerate Medical Research and Drug Development
Disease registries are changing medical research by systematically collecting Real-World Data (RWD)—health information gathered outside of conventional clinical trials. When this data is analyzed to generate insights, it becomes Real-World Evidence (RWE), a powerful tool that is reshaping the entire drug development lifecycle.
RWE captures the complex reality of health, showing how treatments work in diverse, everyday patients who are often excluded from traditional clinical trials due to age, comorbidities, or other factors. This inclusive perspective is essential for optimizing clinical trial design, supporting regulatory decisions, and ensuring treatments are effective for the people who need them most.

Fueling Clinical Trials and Drug Development
Disease registries provide a roadmap for drug developers, particularly in challenging areas like rare diseases.
Registries are the primary source for natural history studies, which track how a disease progresses without intervention. This data is crucial for designing efficient trials by identifying appropriate endpoints, estimating study duration, and understanding patient heterogeneity. Increasingly, natural history data from registries is used to create external control arms (ECAs). An ECA is a group of patients from a registry whose data serves as a comparator for the group receiving the investigational treatment in a clinical trial. This approach is invaluable when a placebo control is unethical or impractical, and regulatory bodies like the FDA and EMA are progressively accepting RWE from ECAs to support drug approvals.
Registries also solve the practical challenge of patient recruitment. They provide an engaged community of potential participants, making it faster and more efficient for researchers to find eligible individuals for trials.
Furthermore, registries are vital for post-market surveillance (Phase IV). After a drug is approved, they monitor its long-term performance and safety in broad, diverse populations. For example, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) identified unexpectedly high failure rates in certain metal-on-metal hip implants. This registry data provided the critical evidence that led to the withdrawal of these faulty products from the market, protecting thousands of patients from harm.
Informing Health Technology Assessment (HTA) and Payer Decisions
Beyond regulatory approval, new treatments must demonstrate value to be covered by insurers and national health systems. RWE from disease registries is essential for this process. Payers and HTA bodies use registry data to assess a drug’s long-term effectiveness, safety, and cost-effectiveness in a real-world setting. This evidence helps them make informed reimbursement decisions and negotiate value-based contracts, ensuring that healthcare resources are allocated to therapies that provide tangible, lasting benefits to patients.
At Lifebit, we’re passionate about making this kind of evidence generation faster and more accessible. You can learn More info about our Real-World Evidence solutions and see how we help transform registry data into insights that save lives.
Supporting Biomarker and Endpoint Development
Disease registries are also treasure maps for scientific findy.
Biomarker findy is a key frontier. Biomarkers are measurable indicators—such as proteins in blood, genetic mutations, or imaging features—that can signal disease status, predict progression, or indicate response to treatment. When registries are linked with biobanks (repositories that store biological samples like blood or tissue), they become exceptionally powerful. This linkage allows researchers to connect rich clinical data from the registry with molecular data from the samples, enabling the findy of novel biomarkers that can lead to earlier diagnoses and personalized treatments.
Crucially for patients, registries help define clinically meaningful endpoints—outcomes that reflect a genuine improvement in a patient’s quality of life, such as reduced pain, better mobility, or increased independence. Patient-Reported Outcome Measures (PROMs), which capture the patient’s perspective directly through surveys and questionnaires, are vital for this. Encouragingly, 57% of registries collect PROMs, signaling a growing recognition that the patient’s experience is the ultimate measure of a treatment’s success.
Building and Managing High-Quality Disease Registries
Creating a successful disease registry is a complex, collaborative effort among diverse stakeholders, including patients, clinicians, researchers, and industry partners, all focused on improving patient outcomes through better data. A solid foundation requires robust governance, clear scientific objectives, and sustainable funding to ensure the collection of high-quality data over the long term.
Key Components for Establishing a Disease Registry
Every successful registry is built on a set of core components that ensure its scientific rigor and operational integrity.
- Clear Objectives: The registry must have a well-defined purpose. Are you tracking natural history, monitoring treatment safety, or improving quality of care? Using a framework like SMART (Specific, Measurable, Achievable, Relevant, Time-bound) goals helps ensure the registry is designed to answer specific, important questions.
- Defined Patient Population: Precise inclusion and exclusion criteria are essential. This defines who is eligible for the registry based on factors like diagnosis, age, and geography. This specificity is critical for collecting a consistent and valuable dataset for research.
- Standardized Data Collection: The registry must define a minimum data set, which is the core information collected from every patient. To ensure data can be compared and combined across different sites and studies, it’s crucial to use Common Data Elements (CDEs). CDEs are standardized questions and data definitions that promote interoperability. Resources like the NIH CDE Repository provide standardized elements for a wide range of research areas.
- Robust Governance Structure: A steering committee or governance board is essential for oversight. This group, which should include clinicians, researchers, and patient representatives, is responsible for setting the registry’s strategic direction, establishing data access policies, and ensuring ethical conduct. A clear charter outlining roles, responsibilities, and decision-making processes is vital.
- Stakeholder Collaboration: No registry succeeds in a vacuum. Clinicians provide frontline expertise, researchers drive scientific inquiry, and regulators ensure evidence meets safety and efficacy standards. Most importantly, patients and advocacy groups must be involved from the beginning to ensure the registry focuses on patient-centric outcomes and builds community trust. Unfortunately, only 38% of registries consult patient advocacy groups during the design phase—a significant missed opportunity for alignment and engagement.
Ensuring Data Quality, Security, and Privacy
For disease registries, trust is the most valuable asset. This trust is built on an unwavering commitment to data quality, security, and patient privacy.
Data Quality and Standardization: High-quality data is the bedrock of credible research. Data standardization is the first step, requiring the use of common coding systems and terminologies like SNOMED CT for clinical findings, ICD-11 for diagnoses, LOINC for lab tests, and RxNorm for medications. This allows data from different sources to be meaningfully combined. Data validation, through automated checks (e.g., range checks, logic checks) and regular audits, acts as a quality control checkpoint to ensure the data is accurate, complete, and consistent.
Data Security and Patient Privacy: Protecting sensitive health information is a non-negotiable ethical and legal obligation.
- Informed Consent: The process must begin with clear and comprehensive informed consent, ensuring patients fully understand what data will be collected, how it will be used, who can access it, and that they have the right to withdraw at any time.
- De-identification: To protect privacy, data is de-identified before being used for research. This can involve anonymization (irrevocably stripping all personal identifiers) or pseudonymization (replacing identifiers with a code, allowing for re-identification if necessary).
- Security Measures: Data must be protected with multiple layers of security, including encryption (both in transit and at rest) and strict access controls that limit who can view information and for what purpose. Researchers typically must sign a Data Use Agreement (DUA) before being granted access.
- Regulatory Compliance: Registries must adhere to strict regulations like the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in Europe, which set high standards for protecting patient data.
At Lifebit, our commitment to these principles shows up in everything we build. Our Trusted Research Environment and Trusted Data Lakehouse create secure spaces where researchers can analyze registry data without ever compromising patient privacy. If you’re curious about how we make this work, you can Learn about secure Trusted Research Environments.
Challenges and Future Trends for Disease Registries
While disease registries are incredibly powerful, running one successfully involves navigating significant challenges, from ensuring long-term sustainability to overcoming technical barriers. Despite these problems, the future holds immense promise, driven by technological innovation and a global shift towards collaborative, patient-centric research.

Overcoming Common Challenges and Limitations
Financial Sustainability
A primary challenge is securing stable, long-term funding. Registries require continuous investment for data collection, curation, technology maintenance, and staffing. Funding is often sourced from short-term research grants, making registries vulnerable. A sobering statistic reveals that less than half of registries report on maintenance plans (46%), putting valuable longitudinal datasets at risk if funding ceases. Sustainable models often involve a diversified approach, combining government grants, industry sponsorship, foundation support, and institutional investment.
Data Quality and Interoperability
Maintaining high data quality and completeness across different collection sites is a constant struggle. Inconsistencies in data entry and missing information can compromise the integrity of research insights. A related barrier is a lack of interoperability. Many registries operate in technical silos, using different data formats and standards. This fragmentation prevents data from being easily shared or combined, limiting the power of large-scale analyses and creating missed opportunities for findy.
Regulatory and Ethical Complexities
Navigating the complex and evolving landscape of data privacy regulations, such as GDPR and HIPAA, is a major undertaking, especially for international research collaborations. Gaining ethical approvals from multiple institutional review boards (IRBs) can be a slow and cumbersome process, delaying important research.
Patient Recruitment and Engagement
Recruiting participants and keeping them engaged over many years is a persistent challenge, particularly for rare diseases with small, geographically dispersed populations. Participants can experience “registry fatigue,” leading to drop-offs and incomplete longitudinal data. Maintaining engagement requires a proactive strategy, including regular communication, sharing research findings back with the community, and demonstrating the registry’s impact.
The Future of Disease Registries: Interoperability and AI
Despite these challenges, the future of disease registries is bright, with new technologies and collaborative frameworks ready to open up their full potential.
A Shift Towards Interoperability and FAIR Data
The adoption of the FAIR Guiding Principles (Findable, Accessible, Interoperable, Reusable) is a critical step forward. The goal is to make registry data findable by the wider research community (Findable), accessible under clear conditions (Accessible), able to be combined with other datasets (Interoperable), and well-described enough to be used in future studies (Reusable). This framework maximizes the value of each dataset.
The Rise of Federated Networks
Federated networks are revolutionizing data sharing by reversing the traditional model. Instead of moving sensitive patient data to a central location, this approach brings the analysis to the data. Researchers send their analytical queries to the distributed datasets within each institution’s secure environment. The analysis runs locally, and only the aggregated, non-identifiable results are returned. This protects patient privacy and institutional control while enabling powerful, collaborative research across global datasets.
Integration of AI and Machine Learning
The integration of AI and Machine Learning (ML) is set to transform registry analysis. These tools can analyze vast, complex datasets to uncover hidden patterns. For example, Natural Language Processing (NLP) can extract structured data from unstructured clinical notes, while predictive models can identify patients at high risk of disease progression or find novel patient subgroups that may respond differently to treatments. This accelerates biomarker findy and paves the way for more personalized medicine.
Empowering Global Collaboration
These technological advances are fueling a new era of global collaboration, especially for rare disease networks. With over 800 rare disease registries in Europe alone, securely connecting these datasets through federated networks creates immense statistical power for findy. This democratization of research empowers patient advocacy groups and individual researchers to take active roles in shaping scientific priorities and accelerating the search for cures.
Our federated platform enables exactly this kind of secure, cross-institutional collaboration. We’re not just talking about the future; we’re building it today, ensuring that every piece of patient data can contribute to the next medical breakthrough while maintaining the trust and privacy that patients deserve.
Frequently Asked Questions about Disease Registries
How can I find a registry for my condition?
Finding a disease registry for your condition can be straightforward if you know where to look. Several reliable resources can connect you with the right one.
- Patient Advocacy Groups (PAGs): This is often the best place to start. PAGs for specific diseases frequently lead, fund, or partner with registries. They can provide trusted information and direct links to participate, especially for rare diseases.
- Your Physician or Specialist: Your treating doctor is an excellent resource. They are often aware of or involved in major registries connected to academic medical centers and ongoing research programs.
- Online Databases: For a broader search, several online portals list registries. Key resources include the NIH’s List of registries from the NIH and, for rare conditions, the registry list at the National Organization for Rare Disorders (NORD).
- Clinical Trial Registries: Websites like ClinicalTrials.gov list observational studies, including many registries, alongside interventional trials.
Is my data safe in a disease registry?
Yes. Data safety and patient privacy are the highest priorities for any reputable disease registry. Your health information is deeply personal, and multiple layers of protection are in place to safeguard it.
- De-identification: Before data is used for research, all personal details that could identify you (like your name, address, or social security number) are removed or replaced with a code. Researchers work with this de-identified data to analyze trends without knowing individual identities.
- Encryption: Your data is encrypted both when it’s being sent (in transit) and when it’s being stored (at rest). This makes it unreadable to any unauthorized parties.
- Strict Access Controls: Only authorized researchers with a legitimate, approved research question can access the data. These controls ensure that data is only used for its intended purpose.
- Legal and Ethical Frameworks: Registries operate under strict legal rules like HIPAA compliance in the U.S. and GDPR compliance in Europe. These regulations impose legally enforceable standards for data protection, with serious penalties for violations.
- Informed Consent: The entire process is governed by informed consent. You will be given clear information on what data is collected, how it will be used, and who might see it. You always have the right to withdraw your consent and your data at any time.
What is the difference between a disease registry and a clinical trial?
This is a common and important question. While both are crucial for medical research, they serve different purposes.
| Feature | Disease Registry | Clinical Trial |
|---|---|---|
| Purpose | Observational: To observe and collect data on what happens in routine care. | Interventional: To test a specific intervention (like a new drug or therapy). |
| Question | Asks “What is happening?” | Asks “Does this treatment work?” |
| Design | Follows a broad group of patients over time during their normal care. | Follows a strict protocol where participants are assigned to specific groups. |
| Data | Collects real-world data that reflects the diversity of patients and care. | Collects highly specific data to answer a narrow research question. |
In short, a registry watches, while a clinical trial tests. They are highly complementary. A registry can provide the natural history data needed to design a clinical trial, help find eligible patients for that trial, and then monitor the long-term safety of the new treatment after the trial is over.
What is the role of a patient advocacy group (PAG) in a registry?
Patient advocacy groups (PAGs) are essential partners in the success of disease registries, particularly for rare conditions. They are the bridge between the patient community and the research world.
- Ensuring a Patient-Centric Focus: PAGs play a critical role in registry design, ensuring that the data collected reflects what truly matters to patients, such as quality of life outcomes, symptom burden, and daily functioning, not just clinical measurements.
- Building Trust and Driving Recruitment: An endorsement from a trusted PAG is invaluable. They act as advocates for the patient community, which encourages participation. Their deep community connections and grassroots outreach are also highly effective for participant recruitment.
- Providing Sustainable Funding: Many PAGs provide critical funding for registries, viewing them as long-term investments in their community’s future and a vital tool for accelerating research.
- Governing and Disseminating Research: PAGs often sit on registry steering committees, ensuring research addresses patient priorities. They also play a key role in sharing findings back with the community, closing the loop between research and the patients who made it possible.
Conclusion
Disease registries have evolved into powerful engines for medical findy, reshaping research and drug development by creating vital bridges between patients, clinicians, and researchers.
The shift towards patient-centric models is particularly striking. The power of collective data, driven by patient advocacy groups and families, offers hope and accelerates research on a global scale. This collaborative spirit is driving the future of collaborative research, moving us from isolated data silos to interconnected networks where new insights can emerge from linking disparate datasets.
At Lifebit, our federated platform enables this secure, cross-institutional analysis of disease registry data. Our approach keeps sensitive patient information at its source while allowing researchers to analyze combined data from multiple registries. We’re accelerating insights while protecting patient privacy, so you don’t have to choose between scientific progress and data security.
Our Trusted Research Environment and federated AI capabilities are already helping researchers connect global datasets to power drug development and public health initiatives.
If you’re ready to see how federated data analysis can transform your research, we can show you what’s possible when we bring computation to the data. Find how federated data analysis can power your research.

