Beyond the Lab Coat: How AI is Supercharging Medical Research

Your Billion-Dollar, 10-Year Drug Trial Is Obsolete. Here’s How AI Cuts Costs by 40%.
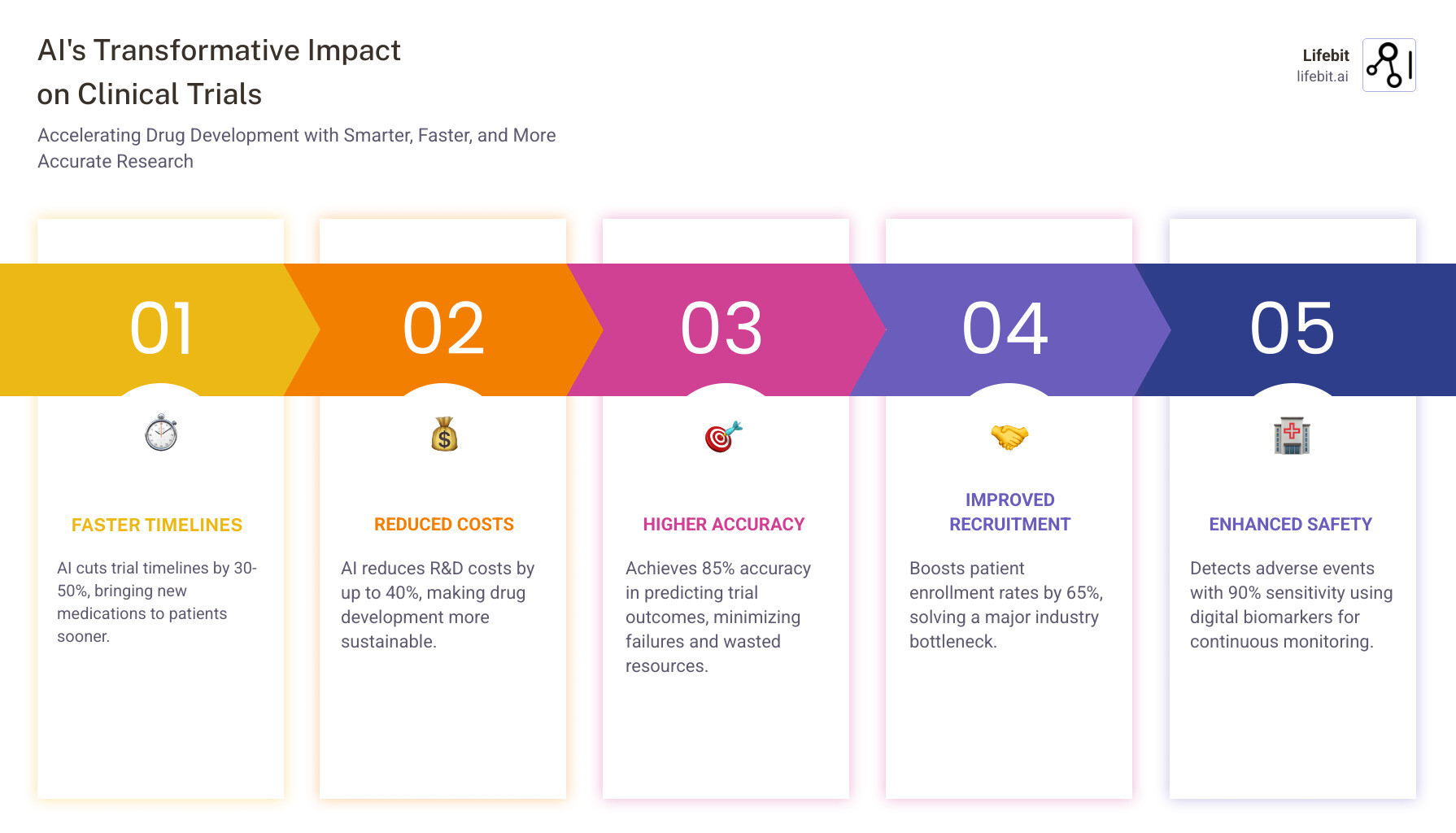
AI-powered medical research is changing clinical trials by accelerating timelines, reducing costs, and improving success rates. Here’s what you need to know:
- Speed: AI cuts trial timelines by 30-50%
- Cost: Reduces R&D costs by up to 40%
- Accuracy: Achieves 85% accuracy in predicting trial outcomes
- Recruitment: Improves patient enrollment rates by 65%
- Safety: Detects adverse events with 90% sensitivity using digital biomarkers
The numbers are staggering. It now takes over a billion dollars and a decade to bring one new medication to market. Half of that time and money gets burned on clinical trials alone. Only one in seven drugs entering phase I trials ever reaches approval. Even worse, pharmaceutical R&D productivity has been declining for 60 years—a phenomenon researchers call “Eroom’s Law” (Moore spelled backwards). The number of drugs approved per billion dollars spent has halved every nine years.
Meanwhile, 80% of clinical trials face recruitment delays. Data quality issues affect half of all datasets. Success rates hover below 12%. And pharmaceutical R&D spending has ballooned past $200 billion annually, with little to show for it.
But artificial intelligence is changing this equation fast. AI can scan electronic health records to find eligible patients in seconds instead of weeks. It predicts which trials will succeed or fail before they burn through millions. Digital biomarkers from wearables monitor patients continuously, catching problems early. Machine learning models analyze genomic data to identify novel drug targets and repurpose existing medications.
The impact is already measurable. AI-powered tools are improving enrollment rates by 65%. Predictive analytics achieves 85% accuracy in forecasting outcomes. Integration of AI into trial workflows accelerates timelines by 30-50% while slashing costs by 40%. These aren’t projections—they’re results from real implementations.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over 15 years building federated AI platforms that enable pharmaceutical companies and public health agencies to open up insights from siloed biomedical data. Through ai-powered medical research, we’re helping organizations run faster, smarter trials while maintaining the highest standards for data privacy and regulatory compliance.
Cut Your Trial Timeline by 50%? How AI Slashes Recruitment Delays and Predicts Success.
Clinical trials eat up half the time and money in drug development. The process is painfully slow, and the failure rate is brutal—only one in seven drugs that enter Phase I trials ever makes it to patients. But this is exactly where ai-powered medical research is making its biggest impact.
We’re watching AI cut trial timelines by 30-50% and slash costs by up to 40%. These aren’t small improvements. They’re the difference between a drug reaching patients in five years versus ten. Between spending $500 million versus nearly a billion.
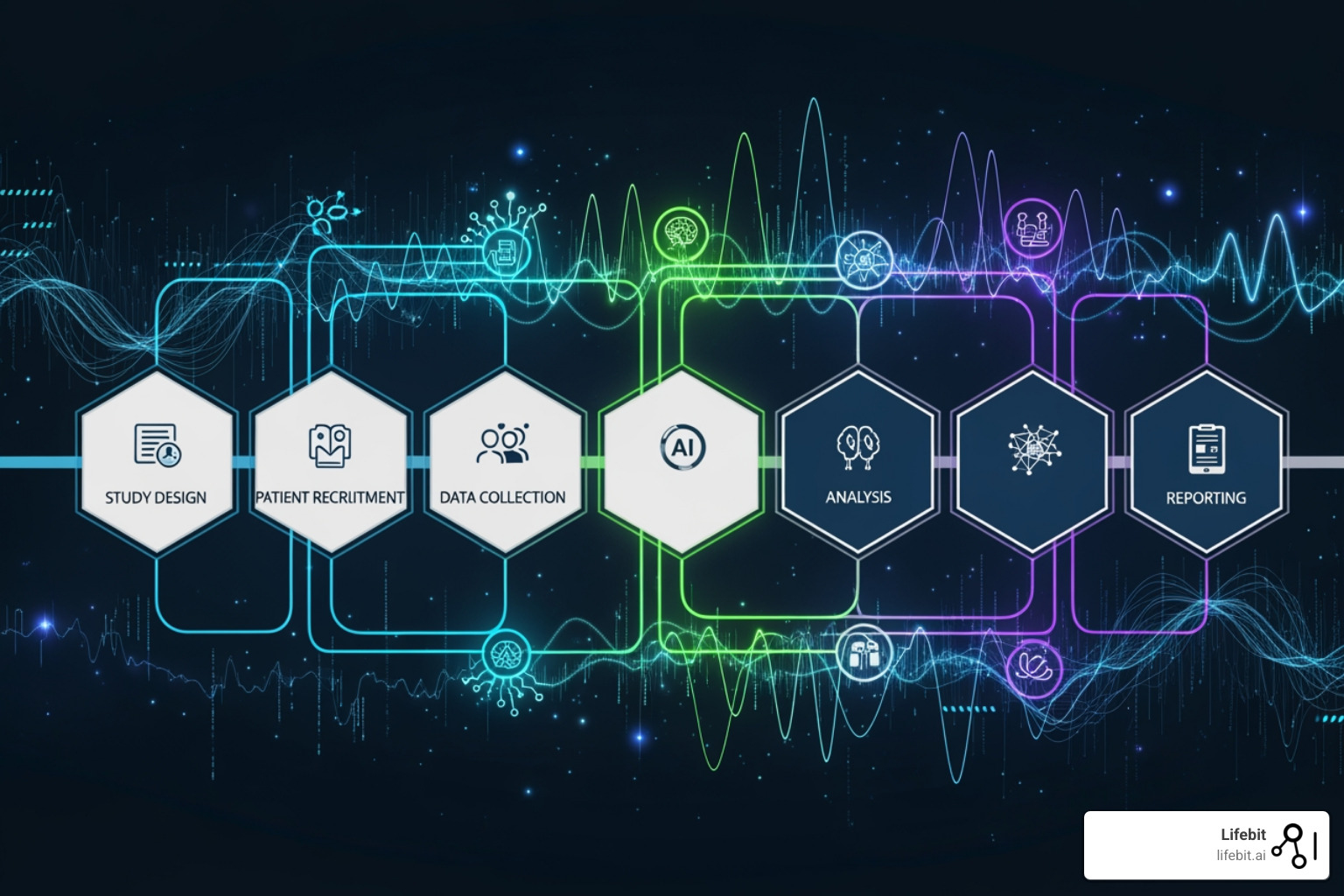
How does AI pull this off? By optimizing trial design from the start. AI can analyze millions of scientific papers, clinical records, and real-world data to identify the most promising approaches before you invest a dollar. It automates protocol development, catching potential issues that might derail a trial months down the road. And it revolutionizes site selection, identifying research centers with the right patient populations and track records.
Think of AI as doing the heavy lifting on all the tedious, time-consuming tasks that don’t require human creativity. This frees up researchers to focus on the truly innovative work—the science that actually moves medicine forward. The result? Faster trials, lower costs, and more drugs reaching patients who need them.
Smarter Recruitment: A Cornerstone of ai-powered medical research
Here’s a sobering statistic: 80% of clinical trials face recruitment delays. Finding the right patients is often the single biggest roadblock to getting a trial across the finish line. A trial can have the best design in the world, but if you can’t enroll enough participants, it’s dead in the water.
This is where Natural Language Processing becomes invaluable. NLP algorithms can scan through thousands of electronic health records in seconds, identifying patients who meet your specific inclusion and exclusion criteria. What would take a human research coordinator weeks or months happens almost instantly. For example, a trial for a new oncology drug might have dozens of complex criteria: a specific genetic mutation, a certain stage of cancer, previous treatment history, and a list of comorbidities that would exclude a patient. Manually sifting through unstructured clinician notes, pathology reports, and lab results for this information is a monumental task. NLP models, however, are trained to understand medical terminology and context. They can extract these specific data points from free-text fields, effectively translating messy human language into structured, queryable data. This not only accelerates the search but also uncovers eligible patients who might have been missed due to data entry variations or overlooked notes.
The impact is measurable. AI-powered patient recruitment tools improve enrollment rates by 65%. But it’s not just about speed—it’s about precision. AI finds more diverse patient populations, ensuring your trial results are actually representative of the real world. The push for diversity is not just an ethical imperative; it’s a scientific one. Drugs can have different efficacy and safety profiles across different ethnic groups due to genetic variations. A trial conducted on a homogenous population may lead to a drug being approved that is less effective or even harmful for underrepresented groups. By systematically scanning vast and varied health record databases, AI can help construct trial cohorts that mirror the true diversity of the patient population, leading to more robust, generalizable, and equitable clinical outcomes. It can identify rare patient subgroups that a human reviewer might miss buried in mountains of medical records.
And AI doesn’t stop at recruitment. It helps with retention too. By analyzing patient data patterns, AI can flag individuals who might be at risk of dropping out. Maybe they’re missing appointments or showing signs of frustration. This early warning gives your team a chance to intervene with additional support before you lose that participant entirely.
As Nature reported, AI is accelerating clinical trials by changing patient acquisition from a bottleneck into a competitive advantage.
Predicting Success with AI-Powered Analytics
Wouldn’t it be great to know if a trial is likely to fail before you’ve spent millions of dollars and years of effort? That’s exactly what predictive analytics delivers.
Our AI models achieve 85% accuracy in forecasting trial outcomes. That means we can spot at-risk trials early, when you still have options. Maybe the protocol needs adjustment. Maybe you need to shift resources to a more promising candidate. Or maybe it’s time to cut your losses and redirect that investment to something with better odds. These predictive models are not crystal balls, but sophisticated statistical engines. They are trained on vast repositories of historical clinical trial data, learning the complex, non-linear relationships between trial design parameters (e.g., endpoint selection, patient criteria), drug characteristics (e.g., mechanism of action, molecular structure), and ultimate trial success or failure. For instance, a model might learn that drugs with a specific binding affinity, tested in a patient population with a particular biomarker, and using a certain primary endpoint, have historically had a 90% failure rate in Phase II. Armed with this insight, a pharmaceutical company can redesign the trial before it begins, perhaps by selecting a different patient subgroup or adjusting the dosage, thereby turning a likely failure into a potential success. This is often referred to as ‘in-silico’ clinical trial simulation, a powerful tool for de-risking development.
Machine learning models analyze complex datasets that no human could process—genetic markers, patient demographics, historical trial data, real-world evidence from previous studies. They find subtle patterns and correlations that predict not just whether a drug will work, but also potential adverse events, optimal dosing strategies, and which patient subgroups are most likely to respond.
This transforms clinical development from a reactive guessing game into a proactive, data-driven process. Instead of waiting for a trial to fail, you can see the warning signs and course-correct. Instead of one-size-fits-all protocols, you can personalize approaches based on what the data actually tells you.
The result? Higher success rates, better resource allocation, and more drugs making it through the pipeline. AI-powered medical research is turning the traditional trial-and-error approach into something far more strategic and efficient.
How AI-Powered Digital Biomarkers Detect Adverse Events with 90% Sensitivity
Modern medicine generates an absolutely staggering amount of data. Every patient visit, lab test, genetic screen, and wearable device reading adds to an ever-growing mountain of information. We’re talking about real-world data (RWD) from everyday healthcare encounters, plus incredibly complex multi-omic data spanning genomics, proteomics, and metabolomics. Traditional analysis methods simply can’t keep up with this tsunami of information.
This is where ai-powered medical research truly comes into its own. Machine learning models excel at making sense of noisy, sparse, and irregular datasets—the exact kind of messy real-world data that confounds conventional approaches. AI can take disparate data types from completely different sources, harmonize them into a coherent whole, and spot subtle patterns that signal disease progression, treatment response, or early warning signs of adverse events.
I won’t sugarcoat it: data quality issues still plague about 50% of clinical trial datasets. But AI is proving remarkably effective at cleaning up this mess, standardizing information across different formats and sources, and even filling in gaps through intelligent augmentation. This transforms data that would otherwise be unusable into a goldmine of actionable insights.
Opening up Insights with Digital Biomarkers
One of the most exciting developments in ai-powered medical research is the emergence of digital biomarkers. These are objective, quantifiable measurements of physiological and behavioral data collected continuously from wearable sensors, mobile devices, and other remote monitoring tools. Think of them as a constant health check-up happening in the background of a patient’s daily life.
The power of this approach is remarkable. Digital biomarkers powered by AI can detect adverse events with 90% sensitivity. That means we can catch problems before they become serious, intervene quickly, and dramatically improve patient safety. Instead of waiting for a patient to report symptoms at their next scheduled visit, we’re monitoring continuously and spotting trouble early.
Wearable sensors and smartwatches generate vast streams of real-time data—heart rate, activity levels, sleep patterns, blood oxygen, and more. AI algorithms process these continuous data streams, identifying deviations from each patient’s baseline, predicting health risks, and providing actionable insights for both clinicians and researchers. For example, in a clinical trial for a new cardiovascular drug, a patient wearing a smartwatch can have their heart rate variability and atrial fibrillation events monitored 24/7. An AI algorithm can analyze this stream to detect subtle, early signs of an adverse cardiac event, like a slight but persistent arrhythmia, that would be completely missed between clinic visits. In neurology, digital biomarkers are transforming the study of diseases like Parkinson’s. By analyzing a patient’s gait and fine motor skills through their smartphone’s accelerometer, AI can quantify disease progression or response to treatment with a level of precision and frequency impossible with traditional clinical assessments. This continuous, objective data provides a much richer and more accurate picture of a drug’s real-world effect on a patient’s quality of life.
This fundamentally shifts healthcare from episodic care (you only get checked when you have an appointment) to continuous, proactive monitoring. The result? We gain an unprecedented window into patient health as it actually happens in the real world, not just in the artificial environment of a clinic visit.
Bridging the Gap from Lab to Clinic
The path from a promising laboratory finding to an effective clinical treatment is notoriously long and fraught with failure. Sobering statistic: only one in seven drugs entering Phase I trials ever receives approval. This enormous translational gap wastes billions of dollars and countless years of effort.
AI-powered medical research is uniquely suited to bridge this chasm. AI accelerates translational research by identifying novel drug targets, repurposing existing drugs for new indications, and advancing personalized medicine. It provides instant insights at the molecular level, making in-silico predictions that guide drug discovery without requiring years of expensive lab work. Drug repurposing is a particularly powerful example. Instead of starting from scratch, AI models can scan databases of existing, approved drugs and predict their potential efficacy against new diseases based on molecular structure, pathway analysis, and genetic associations. For example, an AI might identify that a drug approved for rheumatoid arthritis targets a biological pathway that is also implicated in Alzheimer’s disease, suggesting a new, much faster path to a potential treatment. This approach dramatically lowers the risk and cost, as the safety profile of the existing drug is already well-understood.
AI can identify specific domains in proteins that bind to certain drugs and functionally annotate poorly understood proteins implicated in diseases like Parkinson’s. Tools like AlphaFold have revolutionized protein structure prediction, solving in months what previously took decades of painstaking experimental work. This deeper understanding of disease mechanisms at the molecular level enables us to design far more targeted therapies.
Perhaps most exciting is AI’s ability to enable true personalized medicine. By analyzing vast amounts of genomic and clinical data, AI can predict individual patient responses to therapeutic drugs with over 80% accuracy in many cases. This means we can ensure the right patient receives the right treatment at the right time—no more one-size-fits-all approaches. The promise of personalized medicine extends beyond just matching a patient to a drug. AI can help stratify patients into ‘responder’ and ‘non-responder’ subgroups based on their unique multi-omic profile. This allows for the design of smaller, more targeted, and more successful clinical trials. Instead of a trial failing because the drug only worked for 30% of a broad patient group, a trial can succeed by focusing only on the 30% of patients who are genetically predisposed to respond. This is the essence of precision medicine, and it’s a paradigm shift that is only possible through the analytical power of AI.
AI models can even predict the presence of over 1,000 diseases years before symptoms appear. This early detection capability, as highlighted by research on AI model for early disease detection, opens up entirely new possibilities. Interventions can begin sooner, potentially altering disease progression and dramatically improving outcomes.
By integrating information across genetics, proteins, and clinical outcomes, AI speeds up discoveries and brings us closer to a future where laboratory findings rapidly translate into effective treatments that actually help patients. That’s the promise of bridging the gap from lab to clinic—and AI is finally making it a reality.
AI Bias, Privacy Risks, and Patient Distrust: How to Navigate the 3 Biggest Hurdles in Medical AI
Let me be honest with you: ai-powered medical research isn’t a magic wand that solves everything overnight. Yes, the promise is extraordinary, but the path forward is filled with very real challenges that we need to face head-on.
The technical problems alone are daunting. Healthcare data lives everywhere—scattered across hospitals, clinics, research institutions, each using different systems and formats. Getting all this data to actually talk to each other? That’s what we call the data interoperability problem, and it’s a massive one. Then there’s the cost. Building AI infrastructure that can handle sensitive medical data securely doesn’t come cheap. We’re talking substantial investments in technology, talent, and time.

But the technical challenges pale in comparison to the human ones. This is where things get really complicated, and frankly, where they matter most. Algorithmic bias is a serious problem. Current datasets often reflect existing societal inequities, and if we’re not careful, AI systems trained on this data will bake those biases right into our healthcare system. We’ve already seen medical algorithms that perform poorly on darker skin tones, or scheduling systems that wrongly predict higher no-show rates for Black patients. These aren’t hypothetical problems—they’re happening now.
Then there’s the issue of AI “hallucinations.” Yes, AI systems can make things up and present fabricated information as fact. When you’re dealing with someone’s health, that’s terrifying. And it’s why human oversight isn’t optional—it’s essential.
Patient trust is another delicate balance. While many people are intrigued by AI’s potential in healthcare, about 60% of Americans say they’d be uncomfortable with their doctor relying primarily on AI for their care. That discomfort matters. It tells us that no matter how sophisticated our algorithms become, people still want—and need—that human connection, that human judgment.
The reality is that data privacy and patient consent aren’t just legal checkboxes. They’re fundamental to maintaining the trust that makes medical research possible in the first place. People need to know their most sensitive information is protected, that they understand how it’s being used, and that they have real control over it.
Ensuring Ethical AI: Bias, Privacy, and Trust
Building ethical AI systems starts with acknowledging that our current approach has serious blind spots. When AI models are trained primarily on data from limited, homogenous populations, they don’t work well for everyone else. It’s that simple, and that problematic.
We need to prioritize diverse datasets from the very beginning. This means actively seeking out data that represents different ethnicities, ages, genders, socioeconomic backgrounds, and geographic regions. It’s not enough to add diversity as an afterthought—it needs to be baked into how we design and train our models from day one.
Data privacy requires constant vigilance. Yes, we have frameworks like GDPR in Europe and HIPAA in the USA, but AI systems process data in ways that push these regulations to their limits. Multi-omic data—genomics, proteomics, metabolomics—is incredibly sensitive. A person’s genetic information doesn’t just reveal things about them; it reveals information about their entire family. The stakes for protecting this data couldn’t be higher.
Here’s where the “black box” problem becomes critical. When an AI system makes a recommendation, we need to understand why. Model explainability isn’t just nice to have—it’s essential for building trust with both clinicians and patients. A doctor needs to know the reasoning behind an AI’s suggestion before acting on it. A patient deserves to understand how AI is being used in their care.
Building trust means being transparent about what AI can and cannot do. It means admitting when we don’t have all the answers. It means training people properly to use these tools, understanding both their power and their limitations. When we’re open about AI’s capabilities and its constraints, we create space for it to be a genuine partner in healthcare, not a replacement for human expertise.
Building the Guardrails: The Need for Smart Regulation
The AI revolution in healthcare is moving fast—probably too fast for regulation to keep up easily. But we can’t afford to let innovation race ahead without proper guardrails. The potential consequences are too serious.
Regulatory uncertainty is one of the biggest barriers holding back wider adoption of ai-powered medical research right now. Researchers and companies want to do the right thing, but they need clear rules of the road. That’s why regulatory bodies worldwide are working overtime to establish frameworks that protect patients while still allowing innovation to flourish.
The MHRA in the UK has developed a comprehensive strategy for regulating AI-powered medical devices, focusing on patient safety without stifling innovation. In the USA, the FDA has been actively developing guidelines specifically for AI and machine learning in software as medical devices. The European Medicines Agency is taking similar steps, making AI regulation a strategic priority across Europe.
These aren’t isolated efforts. The AI Governance Alliance, launched by the World Economic Forum, brings together stakeholders from around the world to tackle the uncertainties surrounding AI governance. This kind of global collaboration is crucial because healthcare doesn’t stop at borders—and neither should the standards that keep patients safe.
What we need are regulations that are smart, not just strict. Rules that ensure AI systems are transparent, fair, and accountable, but flexible enough to adapt as technology evolves. At Lifebit, we’ve built our entire platform around this principle—providing the security, governance, and compliance features that enable ai-powered medical research to happen responsibly, at scale, across organizational and geographic boundaries.
The goal isn’t to slow down progress. It’s to make sure that when AI transforms healthcare—and it will—it does so in a way that benefits everyone, protects the vulnerable, and maintains the trust that makes medical research possible.
The Next Frontier: How AI Scientists and Digital Twins Are Automating Medical Discovery
The change we’re witnessing isn’t some distant future—it’s happening right now, and the ripple effects will reshape medicine for generations to come. AI-powered medical research is democratizing access to cutting-edge treatments, addressing critical healthcare gaps, and fundamentally changing how we train the next generation of physicians.
The numbers tell a sobering story. Right now, 4.5 billion people lack access to essential healthcare services. By 2030, we’re facing a projected shortage of 11 million health workers globally. These aren’t just statistics—they represent real people who can’t get the care they need. AI offers a powerful solution to bridge this gap by scaling medical expertise beyond traditional boundaries.
Think about it: AI can provide diagnostic assistance and treatment recommendations in remote clinics that have never seen a specialist. It makes vast amounts of medical knowledge and analytical power accessible to researchers and clinicians regardless of whether they’re in Boston or rural Bangladesh. This is what true democratization looks like—leveling the playing field so that innovation and insight aren’t limited by geography or resources.
The impact on medical education is equally profound. There’s ongoing debate about whether powerful AI technologies might shortcut the learning process for doctors, potentially undermining critical thinking skills. But the reality is more nuanced and ultimately more optimistic. AI won’t replace physicians—it will augment them, much like the internet transformed medicine without eliminating the need for doctors.
Medical students are already recognizing this shift. In one recent study, 71% of medical students believed AI should be integrated into their curriculum. The future of medical training will emphasize data literacy, understanding AI’s capabilities and limitations, and developing the critical thinking skills needed to work effectively alongside these powerful tools. AI can serve as a “second opinion” for clinicians, providing instant insights and helping them tackle tricky cases with greater confidence. It makes good doctors even better.
The Next Frontier of ai-powered medical research
We’re standing at the edge of something extraordinary. The next wave of ai-powered medical research will push far beyond what we’ve accomplished so far, opening doors we barely knew existed.
Generative AI is already showing remarkable promise in hypothesis generation—imagining new drug candidates, designing novel experiments, and even drafting scientific papers. Research teams are exploring the concept of an “AI scientist” capable of fully automated scientific findy, generating ideas, executing experiments, and communicating findings. This isn’t science fiction; it’s active research documented in recent papers.
Tools like PubTator 3.0 are revolutionizing how researchers access biomedical knowledge. This AI-powered literature resource offers semantic and relation searches across millions of articles, connecting proteins, genetic variants, diseases, and chemicals in ways that dramatically improve the accuracy and verifiability of information. When integrated with large language models like GPT-4, it becomes even more powerful, helping researchers find exactly what they need without wading through irrelevant results.
The convergence of AI with quantum computing promises breakthroughs we can barely imagine today. Simulations and analyses currently beyond our reach will become routine. We’re moving toward autonomous trials, where AI manages everything from patient monitoring to data analysis, dramatically accelerating the pace of findy.
Then there’s ambient intelligence in healthcare—AI systems that seamlessly integrate into clinical environments to handle administrative burdens. Tools like Microsoft’s Dragon Copilot already document patient visits automatically, freeing physicians to focus on what matters most: connecting with their patients. This technology enables the creation of “digital twins”—virtual replicas of patients that let us test interventions in a simulated environment before applying them in the real world.
At Lifebit, we’re building the infrastructure that makes all of this possible. Our federated AI platform enables secure, real-time access to global biomedical data while maintaining the highest standards for privacy and compliance. Components like our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer) deliver the real-time insights and AI-driven analytics that power next-generation research.
These advancements won’t just accelerate research—they’ll personalize medicine to an unprecedented degree. Every patient will benefit from treatments designed specifically for their unique biology, informed by insights drawn from millions of similar cases worldwide. That’s the future we’re building, and it’s closer than you think.
The Bottom Line: AI Is Slashing Trial Costs by 40%. Are You Being Left Behind?
The path from laboratory findy to life-saving treatment has long been an expensive, slow, and frustrating journey. But we’re witnessing something remarkable—ai-powered medical research is fundamentally reshaping how we develop and test new medicines.
The change is already measurable and profound. Clinical trial timelines are shrinking by 30-50%. R&D costs are dropping by up to 40%. AI predicts trial outcomes with 85% accuracy, helping us make smarter decisions before millions of dollars are spent. Patient recruitment, once the bane of every trial manager’s existence, is improving by 65%. Digital biomarkers catch adverse events with 90% sensitivity, keeping patients safer than ever before.
But here’s what truly excites me: AI isn’t replacing human expertise—it’s amplifying it. It’s giving researchers superhuman abilities to spot patterns, predict outcomes, and bridge the frustrating gap between promising lab results and effective treatments. We’re finally seeing science fiction become medical fact.
Of course, the road ahead isn’t without bumps. Algorithmic bias remains a real concern. Data privacy must be protected with unwavering vigilance. Regulatory frameworks need to evolve as quickly as the technology itself. These challenges are significant, but they’re not impossible. By bringing together technology developers, clinical researchers, and regulatory experts, we’re building AI systems that are not just powerful, but safe, ethical, and explainable.
At Lifebit, this is precisely the future we’re building. Our federated AI platform breaks down data silos while maintaining the highest security standards, giving researchers secure, real-time access to global biomedical data. We’re enabling faster, smarter studies that comply with every regulatory requirement. Through our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), we deliver real-time insights, AI-driven safety surveillance, and secure collaboration across complex data ecosystems.
The future of medicine isn’t just about AI doing more—it’s about AI helping us do better. It’s about accelerating breakthroughs, personalizing treatments, and ultimately getting life-changing therapies to patients who desperately need them. And that future? It’s already here.
Ready to see how federated AI can transform your research? Learn how federated AI is transforming research and find what’s possible when cutting-edge technology meets human ingenuity.


