Go Digital: Smart Strategies for Clinical Trial Patient Recruitment

Why Clinical Trials Are Failing to Find Patients—and How to Fix It
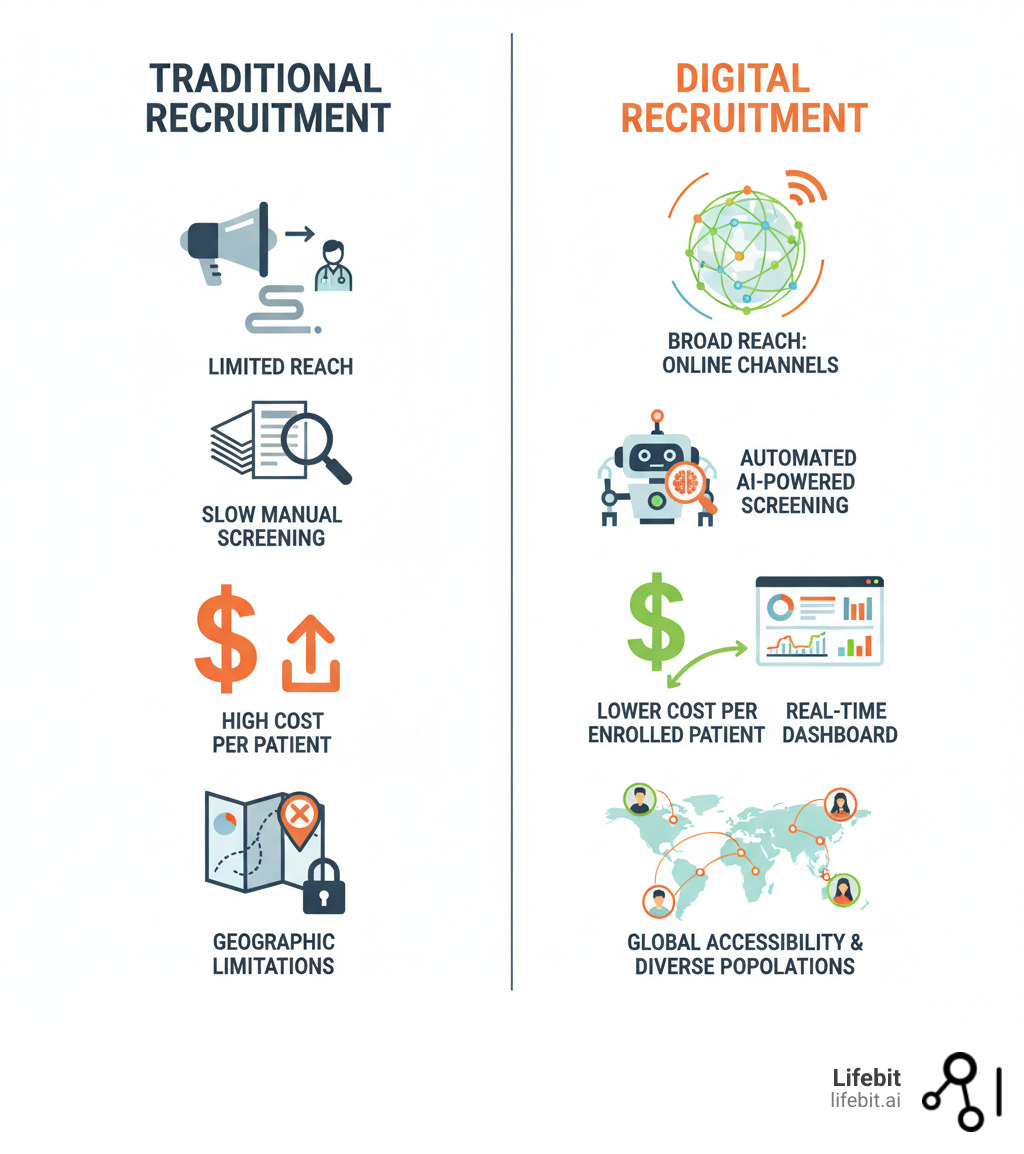
The numbers don’t lie: 80% of clinical trials miss their enrollment deadlines, and these delays cost sponsors up to $1 million per day. With recruitment absorbing 30% of research timelines, the industry’s reliance on outdated methods is no longer sustainable. Traditional approaches, such as physician referrals, print advertising, and manual chart reviews, are failing because they are slow, inefficient, and geographically constrained. Physician referrals are limited by a doctor’s awareness and patient load, while manual reviews of health records are labor-intensive, prone to human error, and simply cannot scale to meet the demands of modern research.
This inefficiency has a profound human cost, delaying access to potentially life-saving therapies. Patients today are proactive and digitally savvy. They expect the same convenience from healthcare that they get from banking or retail. They research trials online, compare options in patient communities, and demand transparency and speed. This is where digital patient recruitment becomes essential. It is a paradigm shift that uses online tools, data analytics, and AI to identify, engage, and enroll patients with unprecedented efficiency. Key strategies include:
- Targeted Digital Advertising: Using search and social media platforms to reach specific patient demographics who are actively searching for health information or fit a trial’s criteria.
- AI-Powered EHR Analysis: Leveraging artificial intelligence to scan vast electronic health record (EHR) databases to find eligible patient cohorts in minutes, not months.
- Online Screening and Pre-Qualification: Deploying intelligent online forms that allow patients to check their own eligibility instantly, reducing friction and capturing interest at its peak.
- Patient Engagement Platforms: Utilizing mobile apps and patient portals to keep participants informed, engaged, and connected throughout the trial journey.
- Support for Decentralized Trials (DCTs): Enabling remote participation through technologies like eConsent, telehealth, and wearable devices, which removes geographic barriers entirely.
By leveraging these tools, organizations are seeing transformative results, including 156% increases in qualified patient inquiries and recruitment timelines cut by an average of 4.2 months. This acceleration is not just a business advantage; it’s a critical step toward getting new treatments to the people who need them faster.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, we’ve built federated data platforms that enable secure, AI-powered digital patient recruitment across siloed healthcare datasets. Our work helps global pharma and public sector leaders find eligible cohorts faster and accelerate life-saving research without moving sensitive patient data.
The Digital Revolution in Patient Recruitment
Patients have changed. If your recruitment strategy hasn’t, you’re already behind. Today’s patients are ‘digital-first.’ They don’t wait for a doctor’s referral; they research their condition online, compare treatments in social media groups, and expect to manage their health from their phones. When a trial’s first impression is a clunky website, an unreturned phone call, or a confusing paper form, potential participants are lost for good. The shift to digital patient recruitment is a fundamental change in how patients find and join clinical trials. The organizations winning at enrollment are meeting patients where they already are: online.
| Metric | Traditional Recruitment | Digital Recruitment |
|---|---|---|
| Cost per Enrolled Patient | $5,000–$8,000 | $1,500–$3,500 |
| Speed to First Patient | 6–9 months | 2–4 months |
| Geographic Reach | Limited to local area | National or global |
| Diversity | Often homogeneous | Broader demographic access |
Key Digital Channels and Strategies
To find these digital-first patients, you need a smart, multi-channel strategy that builds awareness, trust, and accessibility.
- Clinical trial registries: Platforms like ClinicalTrials.gov have evolved from static listings into dynamic search tools for motivated patients. Modern strategies involve optimizing trial listings with clear, patient-friendly language and keywords to improve search visibility, making it easier for patients to find relevant studies.
- EHR-based alerts: This is a powerful form of proactive recruitment. With proper safeguards and patient consent, AI algorithms can scan electronic health records for specific criteria (e.g., diagnosis codes, lab values, prescriptions) and automatically flag potentially eligible patients for follow-up by clinical staff. This transforms recruitment from a passive to an active process.
- Content marketing and patient education: Most trial protocols are filled with impenetrable jargon. AI-driven tools can translate complex medical language into plain-language summaries. This content can be repurposed into blog posts (“What to Expect in a Phase 3 Oncology Trial”), short videos, or infographics that explain the trial’s purpose and what participation involves. This educational approach empowers patients and builds trust.
- Patient advocacy groups: These groups are trusted pillars in their communities. Partnering with organizations like the Michael J. Fox Foundation (for Parkinson’s) or the Sickle Cell Disease Association of America allows sponsors to share trial information through a credible, respected channel. This often involves co-creating educational materials or webinars to ensure the information is accurate, sensitive, and addresses the community’s specific concerns.
- Online health communities: Websites like PatientsLikeMe or disease-specific Facebook groups offer direct access to engaged patients. Authentic, non-promotional engagement—such as answering questions or sharing helpful educational content—can build goodwill and open powerful, community-driven recruitment channels.
How Decentralized and Hybrid Trials Expand Access
Decentralized and hybrid clinical trials are revolutionizing participation by bringing the trial to the patient. Traditional trials exclude anyone who doesn’t live near a major research center or can’t afford the time off work and travel costs for frequent visits. Decentralized Clinical Trials (DCTs) dismantle these barriers by using a suite of digital technologies to enable remote participation.
The technology stack for a DCT often includes:
- Telehealth platforms for virtual visits with investigators.
- Wearable sensors (like smartwatches or continuous glucose monitors) to collect real-world data passively.
- ePRO (electronic Patient-Reported Outcomes) apps for patients to report symptoms and quality of life from their own devices.
- Digital consent (eConsent), which allows participants to review and sign forms electronically on their own time.
- Direct-to-patient logistics for shipping study medication and collection kits directly to a patient’s home.
Suddenly, a patient in a rural area has the same access as one in a major city. This technology becomes the “direct connect” to patients, keeping them engaged while dramatically reducing the burden of participation. When you remove geographic and logistical barriers, you don’t just make recruitment easier—you make it possible to build diverse, representative study populations that reflect the real world. At Lifebit, our federated platform enables sponsors to identify these eligible cohorts across distributed healthcare systems, reaching patients wherever they are without compromising data security.
The Power of Data: AI and Analytics in Digital Patient Recruitment

Finding the right patients for clinical trials is evolving from guesswork to a precision science, thanks to artificial intelligence. The global AI in clinical trials market is projected to reach US$2.74 billion by 2030, reflecting an industry-wide shift toward data-driven recruitment that is faster, more accurate, and more efficient.
Leveraging AI for More Effective Recruitment
AI transforms the frustrating, multi-step screening process into a seamless digital experience. Automated eligibility checks via intelligent online screeners can instantly validate inclusion/exclusion criteria. For example, a screener can use conditional logic to ask relevant follow-up questions based on a patient’s answers, immediately informing them if they are a potential match. This provides real-time feedback and reduces the friction that causes patients to abandon the process.
Another major barrier is confusion. Clinical protocols are often written in dense medical jargon that alienates patients. AI-based summarization tools can translate complex criteria into plain English, making trials more accessible and understandable. When patients clearly understand what a trial involves, why it’s important, and what is expected of them, they are far more likely to participate.
Furthermore, AI enables proactive recruitment by mining electronic health records (EHRs) at scale. Natural Language Processing (NLP) algorithms can scan millions of unstructured clinical notes, discharge summaries, and pathology reports to identify concepts and patient attributes that structured data fields would miss. This allows researchers to find highly specific patient cohorts—a task that would take teams of humans years to complete. At Lifebit, our federated platform enables this intelligent analysis while keeping sensitive patient data secure and unmoved, allowing our AI to identify cohorts across global locations without centralizing or exposing patient information.
Data-Driven Approaches to Finding the Right Patients
Effective digital patient recruitment starts with knowing who you’re looking for and where to find them.
- Predictive Analytics for Site Selection: Before a trial even begins, AI can analyze real-world data (RWD)—including claims data, prescription data, and demographic information—to identify geographic hotspots with the highest concentration of eligible patients. This data-driven approach helps sponsors avoid the costly mistake of opening trial sites in locations with insufficient patient populations.
- Search Behavior Analysis: By analyzing anonymized search engine data, researchers can understand the keywords, questions, and phrases patients use when looking for information about their condition. This insight allows for precisely targeted outreach and content that directly addresses patients’ needs and concerns.
- AI-Powered Ad Optimization: Digital advertising platforms can use AI to automate and optimize campaigns in real time. Algorithms can A/B test different ad creatives, headlines, and targeting parameters to identify which combinations deliver the highest number of qualified leads at the lowest cost, maximizing the recruitment budget.
- Data-Driven Patient Personas: AI can analyze treatment histories, comorbidities, and even social determinants of health to create detailed patient personas. This allows for hyper-personalized messaging that speaks to a patient’s unique situation and concerns, leading to significantly higher response rates and better-quality leads.
Lifebit’s Trusted Research Environment (TRE) and federated architecture make this possible, bringing AI-powered analytics to the data’s location while maintaining full compliance with regulations like HIPAA and GDPR. The future of recruitment is about finding the right patients faster, and that future is already here.
Designing a Patient-Centric and Inclusive Journey
Finding patients is only half the battle. A successful digital patient recruitment strategy requires designing an end-to-end journey that is seamless, supportive, and respectful of each patient’s time and experience. This patient-centric approach uses design thinking principles to understand and eliminate friction at every touchpoint, from initial awareness to final study visit.
Patient journey mapping is a critical exercise to visualize and improve this process. Consider a modern digital journey: a 45-year-old woman with Crohn’s disease sees a targeted ad on social media. She clicks to a mobile-friendly landing page featuring a short video of the lead investigator explaining the trial’s goals in simple terms. She completes a 5-minute pre-screener on her phone and immediately receives a notification that she may be eligible. Within 24 hours, she gets an email to schedule a telehealth consultation. This experience is fast, transparent, and empowering. Contrast this with a traditional journey involving a confusing flyer, a game of phone tag with a study coordinator, and a long wait for an in-person appointment.
Since most patients discover trials on their phones, mobile-first design is non-negotiable. All websites, forms, and communications must be optimized for small screens. Digital consent (eConsent) further streamlines one of the most intimidating steps. Instead of dense paper documents, patients can review interactive forms with embedded definitions and videos, and then sign remotely at their own pace. This isn’t just about convenience—it’s about promoting autonomous, informed decision-making.
After enrollment, continuous engagement tools are key to reducing the average 25% dropout rate. Patient portals, wearables that track data automatically, telehealth apps for check-ins, and automated reminders create a supportive ecosystem that keeps participants connected and motivated.
Ethical Best Practices for Digital Patient Recruitment
With powerful digital tools come significant ethical obligations. Trust is the currency of clinical research.
- Data Privacy and Security: Foundational compliance with regulations like HIPAA and GDPR is the minimum standard. This requires robust encryption, secure platforms, and de-identification of sensitive data wherever possible. Lifebit’s federated environments are designed for secure, compliant research without ever moving or exposing raw patient data.
- Radical Transparency: Patients need clear, honest information about the trial’s purpose, risks, benefits, time commitment, and potential for a placebo. This includes being upfront about how their data will be used and protected. Trust is built when patients feel respected, not just recruited.
- Truly Informed Consent: Digital consent must be even more comprehensive and understandable than paper-based processes. Using interactive elements, quizzes, and direct lines to study staff for questions ensures genuine comprehension, not just a signature.
- Managing Expectations: Clear communication about what participation entails—including the use of digital tools, frequency of communication, and data collection methods—builds confidence and prevents surprises that can erode trust and lead to dropouts.
Using Digital Strategies to Improve Diversity and Inclusion
The persistent lack of diversity in clinical trials is a scientific and ethical failure, limiting the generalizability of new treatments. Digital strategies offer a powerful toolkit to address this.
- Targeted Outreach: Digital advertising allows for granular targeting to reach underrepresented populations with localized, customized messaging.
- Culturally Competent Messaging: This goes beyond simple translation. For a diabetes trial targeting a specific community, for example, messaging might focus on family health and be delivered through trusted local online forums, rather than using generic medical language. This demonstrates respect and relevance, which has been shown to significantly increase enrollment.
- Multi-language Support: All patient-facing digital assets—from the website to the eConsent form to the mobile app—must be available in multiple languages to be truly accessible in diverse regions like Europe and North America.
- Bridging the Digital Divide: A digital-first approach must not become a digital-only barrier. To ensure equity, hybrid strategies are crucial. This can include providing participants with pre-configured tablets or smartphones, offering tech support hotlines, or partnering with community centers and libraries to provide access and assistance. As highlighted in a recent review on leveraging digital tools to enhance diversity, the path forward requires a genuine commitment to health equity.
Measuring Success and The Future of Recruitment
Measuring the success of digital patient recruitment is crucial for optimizing campaigns, demonstrating ROI, and making data-driven decisions. This requires moving beyond simple enrollment numbers to a more granular analysis of performance across the entire recruitment funnel.
Key Metrics for Your Digital Campaigns
Effective measurement requires tracking several Key Performance Indicators (KPIs):
- Cost per Lead (CPL) & Cost per Enrolled Patient (CPE): These metrics track the cost to generate an inquiry versus a successfully enrolled participant. Why it matters: They provide a clear financial measure of campaign efficiency and help justify budget allocation.
- Enrollment & Screen Failure Rates: This is the percentage of qualified leads who ultimately enroll, and the percentage of screened patients who are deemed ineligible. Why it matters: A high screen failure rate may indicate that ad targeting or pre-screener questions are not specific enough, allowing for early course correction.
- Time to Enrollment: The average duration from a patient’s initial contact to their official enrollment. Why it matters: This directly measures the speed of your recruitment funnel. Digital methods can shorten this from months to weeks, accelerating overall trial timelines.
- Conversion Rates by Channel: This analyzes which digital channels (e.g., social media, search ads, patient communities) are most effective at converting leads into enrollments. Why it matters: This tells you where to allocate your budget. If one channel drives high traffic but low enrollment, resources can be shifted to better-performing channels.
- Patient Demographics & Dropout Rates: Tracking the diversity of the enrolled population and the percentage of participants who withdraw from the study. Why it matters: This helps assess whether diversity goals are being met and identifies potential issues with patient retention that need to be addressed.
- Return on Investment (ROI): A comprehensive analysis of the financial benefits (e.g., savings from shorter timelines) versus the costs of the digital campaign. With digital recruitment boosting inquiries by 156% and shortening timelines by 4.2 months, the ROI is substantial.
Future Trends and Innovations in Digital Patient Recruitment
The future of digital patient recruitment is being shaped by continuous technological advancements that promise even greater precision, personalization, and trust.
- Hyper-Personalization: Advanced AI will move beyond demographic targeting to enable communication tailored to an individual’s specific health profile, communication preferences, and even motivational drivers, making outreach feel more like a personal invitation.
- Blockchain for Data Integrity: Blockchain technology offers a decentralized, immutable ledger for clinical trial data. How it works: Every key event, such as a patient giving consent or a researcher accessing data, is recorded as a secure, time-stamped block that cannot be altered. This creates a transparent and verifiable audit trail, enhancing data integrity and building trust among patients, sponsors, and regulators.
- Advanced AI Models: Sophisticated NLP will provide deeper insights from unstructured patient data, while generative AI will create dynamic, personalized patient experiences. For example, a GenAI-powered chatbot could provide 24/7 support, answering a patient’s specific questions about a trial by referencing the protocol in real-time to ensure accuracy.
- Gamification: Incorporating game-like elements—such as progress trackers, badges, and rewards—into patient engagement platforms can boost motivation, improve adherence to study protocols, and make participation feel more rewarding.
- Virtual Reality (VR) for Trial Education: VR can offer immersive, interactive experiences for potential participants. Imagine a patient virtually touring a study site, visualizing how a new drug works in the body, or walking through complex procedures. This can significantly improve comprehension, reduce anxiety, and empower more informed decision-making.
Frequently Asked Questions about Digital Patient Recruitment
How much does digital patient recruitment cost compared to traditional methods?
While costs vary based on trial complexity and therapeutic area, digital patient recruitment typically delivers a significantly lower cost per enrolled patient (CPE) and a stronger overall ROI. Traditional recruitment can consume up to 40% of a trial budget, with delays costing sponsors up to $1 million per day. Digital methods generate savings in several key areas: precision targeting reduces wasted ad spend, automated screening reduces manual labor hours, and the broader reach of online channels can reduce the need to open and maintain numerous underperforming physical trial sites. The efficiency gains and accelerated timelines provide long-term savings that far outweigh the initial investment in technology and expertise.
What are the biggest regulatory problems for digital recruitment?
Navigating the regulatory landscape is a primary concern, but it is manageable with the right expertise. The main challenges include:
- IRB/Ethics Committee Approval: All digital assets—including social media ads, landing pages, pre-screeners, and eConsent platforms—require rigorous review and approval. This often includes submitting a detailed social media plan that outlines how the study team will manage public comments, handle potential adverse event reporting, and ensure all communications are non-coercive.
- Data Privacy Laws: Global trials require a robust strategy to comply with a complex web of regulations, such as HIPAA in the USA, GDPR in Europe, and other national data protection laws. This requires a deep understanding of rules regarding data transfer, storage, and patient rights across different jurisdictions.
- Compliant Communication: All patient-facing messages must be clear, honest, and balanced. They must avoid making therapeutic claims or overstating benefits, adhering to strict regulations on direct-to-consumer advertising for clinical research. Technology platforms with built-in compliance guardrails are essential for mitigating these risks.
How can we ensure data from digital sources is secure and private?
Protecting patient data is paramount and requires a multi-layered, defense-in-depth strategy. Encryption for data in transit and at rest is the foundational layer. All data should be handled on secure, validated platforms that undergo regular security audits and penetration testing.
De-identification and role-based access controls are critical for minimizing risk. Personal identifiers should be removed or pseudonymized whenever possible, and access to data should be strictly limited to authorized personnel on a need-to-know basis.
However, the most advanced approach is the use of federated data environments. This technology allows AI models to be trained on data without the data ever leaving its secure location (e.g., a hospital’s firewall). The analysis travels to the data, not the other way around. The AI model is sent to each data source, it learns from the local data, and only the aggregated, anonymized insights are combined centrally. Lifebit’s federated AI platform was built for this exact challenge. It enables secure, real-time analysis of global biomedical data with built-in governance, powering digital patient recruitment at scale without ever compromising patient privacy.
What skills or team members are needed to run a successful digital patient recruitment campaign?
A modern recruitment team requires a multidisciplinary blend of clinical, marketing, and data science expertise. A high-performing team often includes:
- Digital Marketing Strategist: Manages paid advertising campaigns (search, social), SEO, and overall channel strategy.
- Content Creator / Medical Writer: Translates complex protocols into patient-friendly language for websites, blogs, videos, and ads.
- Data Analyst: Tracks KPIs, manages analytics dashboards, and provides insights to optimize campaign performance and budget allocation.
- Patient/Clinical Liaison: Acts as the bridge between the marketing efforts and the clinical team, ensuring all materials are accurate, ethical, and patient-centric.
- Regulatory & Compliance Specialist: Reviews and approves all patient-facing materials to ensure they meet IRB, ethics, and legal standards.
This collaborative structure ensures that recruitment efforts are not only effective from a marketing perspective but also clinically sound, ethical, and compliant.
Conclusion: Your Next Step in Modernizing Trial Recruitment
If there’s one takeaway, it’s this: digital patient recruitment is no longer an optional upgrade. It’s how modern clinical trials succeed. Traditional methods can’t match the speed, precision, and reach that digital strategies deliver.
The benefits are clear: faster enrollment, broader reach, lower costs, and access to diverse populations that older methods miss. For an industry where 80% of trials miss enrollment deadlines and delays cost millions daily, these improvements are essential for survival.
Of course, speed only matters if the ethics are right. Data privacy, transparency, and informed consent must be at the heart of every digital strategy. The future is already taking shape with hyper-personalization, blockchain for data integrity, and advanced AI models that will predict not just eligibility, but who is most likely to complete a trial.
At Lifebit, we built our platform for this new era. Our federated AI technology enables secure analysis across global datasets without moving sensitive patient information. Whether your data is in London, New York, or across multiple continents, our Trusted Research Environment (TRE) delivers the insights you need while maintaining the highest standards of privacy and compliance with regulations like HIPAA and GDPR.
Your next step is to accept these digital strategies before your competitors do. The trials that succeed will be the ones that meet patients where they are and make participation convenient and trustworthy.
Discover how Lifebit’s federated platform accelerates research and powers a new generation of secure, efficient, patient-centric clinical trials. The future of digital patient recruitment is data-driven, AI-enabled, and built on trust. Let’s build it together.

