Detailed Reviews of the Top 10 Companies in AI Drug Development
Which Companies Are Leading the Way in Using AI in Drug Development? Cut R&D Timelines by 80%
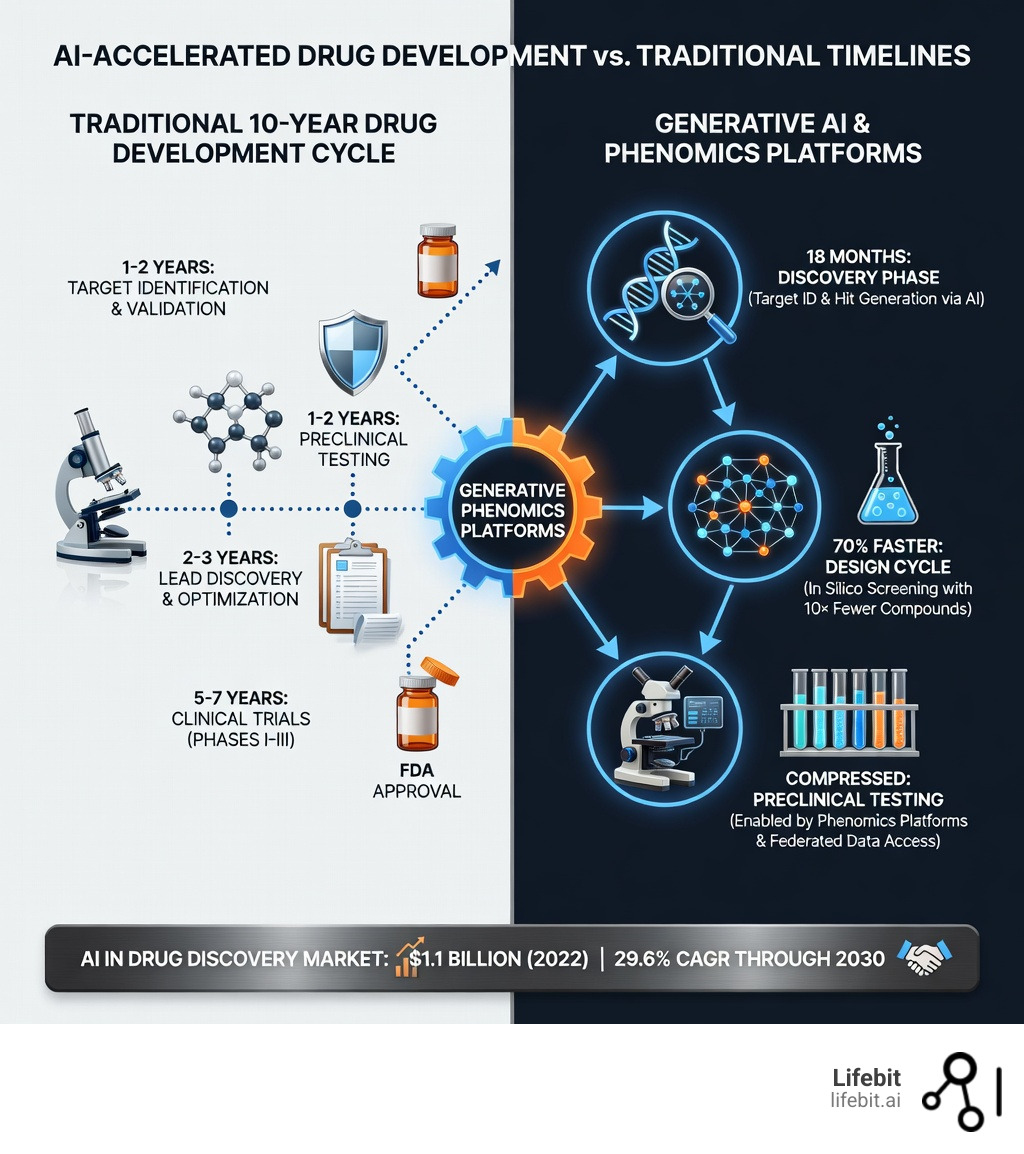
Which companies are leading the way in using AI in drug development? The pharmaceutical industry faces a $236 billion revenue cliff through 2030 from expiring patents, while drug development costs have ballooned past $1 billion per approved drug. AI is now the critical lever that’s compressing 10-year timelines into 18 months and cutting R&D costs by half.
Instead of naming specific vendors or startups, this article breaks down the 10 archetypes of companies leading the way in using AI in drug development and what measurable outcomes they are driving across the pipeline. These archetypes represent the vanguard of a shift from “discovery by chance” to “discovery by design.”
Top 10 Archetypes Leading AI in Drug Development (and what they’re proving):
-
Generative, end-to-end drug design companies – These organizations are proving that the traditional “valley of death” between target identification and clinical entry can be bridged. By using generative models to design molecules with specific properties from scratch, they are demonstrating findy-to-IND (Investigational New Drug) timelines compressed to roughly 18-24 months. This is a radical departure from the industry standard of 5-7 years for the same phase.
-
AI-first medicinal chemistry platforms – These companies focus on the iterative cycle of Design-Make-Test-Analyze (DMTA). By reporting 70% faster in silico design cycles, they materially reduce the number of synthesized compounds per iteration. Instead of synthesizing 5,000 compounds to find a lead, they may only need 500, drastically reducing laboratory overhead and waste.
-
Knowledge-graph and causal inference companies – By aggregating millions of scientific papers, clinical trial results, and patent filings into massive knowledge graphs, these companies identify hidden relationships between biological entities. This allows for rapid target and repurposing hypothesis generation, cutting the time required for prioritization from months of manual literature review to just days of computational querying.
-
Structure and protein-modeling companies – Building on breakthroughs like AlphaFold, these companies use learned representations of protein structure and dynamics. They don’t just look at static snapshots of proteins but model how they move and interact in a cellular environment, which is critical for improving hit finding and lead optimization for complex targets.
-
Generative protein design companies – Moving beyond small molecules, these leaders are creating novel binders and therapeutic proteins that do not exist in nature. By exploring the vast “sequence space” of amino acids, they can design biologics with higher affinity and lower immunogenicity than those derived from traditional animal immunization methods.
-
Deep-learning virtual screening companies – These companies are scaling hit identification across hundreds of targets simultaneously. Using massive GPU clusters, they can screen billions of virtual molecules against a target in a weekend—a feat that would take years using physical high-throughput screening (HTS) in a wet lab.
-
Dynamics-aware small-molecule design companies – Many diseases involve “undruggable” proteins that lack obvious binding pockets. These companies model protein motion and conformations to identify “cryptic” sites that only open up momentarily, allowing for the design of drugs against historically difficult targets like KRAS or certain transcription factors.
-
Disease-omics and patient-data-first companies – These leaders prioritize human biology over animal models. By using human tissue samples and multi-omics (genomics, transcriptomics, proteomics), they link targets to real human biology earlier in the process, which is expected to significantly reduce the high failure rate seen in Phase II clinical trials.
-
Longevity and systems-biology companies – These organizations map the complex pathways of aging and metabolism. Rather than targeting a single protein, they look at the entire system to identify intervention points and biomarkers that can predict how a patient will age or respond to metabolic stressors.
-
Generative antibody and biologics engineering companies – Optimizing antibody candidates for “developability” is a major hurdle. These companies use AI to ensure that a potent antibody can also be manufactured at scale, remains stable in a vial, and has the right pharmacokinetic profile, preventing late-stage failures due to manufacturing issues.
The global AI in drug findy market reached $1.1 billion in 2022 and is expanding at 29.6% CAGR through 2030. By 2024, over 75 AI-derived molecules entered clinical trials—up from zero in 2020. Early programs report Phase I success rates of 80-90% versus the industry average of 40-65%. This suggests that AI is not just making the process faster, but also more accurate in selecting candidates that are safe for humans.
I’m Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, a federated genomics and biomedical data platform powering AI-driven drug findy for global pharmaceutical organizations. With over 15 years in computational biology, AI, and health-tech entrepreneurship, I’ve worked directly with teams leading the way in using AI in drug development to accelerate precision medicine through secure, compliant data environments. Here’s what makes these 10 approaches stand out—and how platforms like Lifebit enable them to move faster.
Learn more about Which companies are leading the way in using AI in drug development?:
$350 Billion at Stake: Why AI-Driven Drug Findy Can’t Wait
The pharmaceutical industry is currently in an “AI arms race.” With a projected $350 billion in annual value up for grabs, the shift from traditional “serendipitous” findy to data-driven engineering is no longer optional. According to Grand View Research, the global AI in drug findy market was valued at $1.1 billion in 2022 and is set to explode at a CAGR of 29.6%. This growth is fueled by the realization that the “Eroom’s Law”—the observation that drug discovery is becoming slower and more expensive despite technological gains—can only be reversed through the industrialization of biology via AI.
Why the sudden urgency? It’s simple: the old way is broken. R&D expenses have increased tenfold since the 1980s, yet success rates remain abysmally low. Only about 10% of drugs that enter clinical trials ever reach the market. Today, a recent study found that AI adoption among researchers has surged from 57% to 84% in just one year. This jump reflects a “reality check” where researchers realize that without AI, they cannot process the petabytes of multi-omic data required to understand modern disease complexity.
Technological advancements like generative AI, deep learning, and phenomics are enabling this change. For example, generative-AI-designed drugs for conditions like idiopathic pulmonary fibrosis (IPF) have moved from target findy to Phase I in just 18 months—a process that typically takes five years. Furthermore, the integration of Large Language Models (LLMs) is allowing scientists to query vast databases of unstructured clinical notes, turning previously “dark data” into actionable insights for trial design and patient stratification.
How Lifebit Powers the Fastest AI Drug Pipelines
At Lifebit, we’ve seen that the biggest bottleneck in AI drug development isn’t the algorithm—it’s the data. Most pharmaceutical companies are sitting on mountains of legacy data that are siloed, unharmonized, and inaccessible. Furthermore, the most valuable data—real-world evidence and genomic data from national biobanks—is often restricted by strict residency laws. We provide the federated AI platform that bridges this gap, allowing researchers to run advanced analytics on global biomedical data without moving it.
| Capability | Traditional Approach | Lifebit-Accelerated AI |
|---|---|---|
| Data Access | Months of legal/IT problems | Secure, real-time federated access |
| Target Findy | Hypothesis-driven (slow) | Data-driven, multi-omic (fast) |
| Molecular Design | Trial and error in labs | In silico optimization via AI/ML |
| Regulatory Compliance | Manual audits and risk | Automated governance and TDL |
| Data Sovereignty | Data must be moved/copied | Data stays in situ (Federated) |
Lifebit’s Trusted Research Environment: The Backbone of AI Drug Development
Our Trusted Research Environment (TRE) is designed to handle the complexity of modern drug findy. It allows researchers to integrate diverse datasets—from UK Biobank genomics to real-world clinical evidence—within a secure, compliant workspace. The TRE operates on the “Five Safes” framework: Safe People, Safe Projects, Safe Settings, Safe Data, and Safe Outputs. This ensures that while AI models learn from the data, the privacy of the individual patients is never compromised.
By providing built-in capabilities for harmonization (using standards like OMOP CDM) and advanced AI/ML analytics, we enable teams to identify novel targets with unprecedented precision. For instance, a researcher can run a Genome-Wide Association Study (GWAS) across multiple international cohorts simultaneously, identifying rare genetic variants that are invisible in smaller, localized datasets.
Case Study: Cutting Drug Findy Timelines in Half with Lifebit
We recently partnered with a top-10 global pharma company that was struggling to integrate multi-omic data across its international research sites. The challenge was twofold: the data was too large to move, and local regulations in several countries prohibited data export. By deploying Lifebit’s federated platform, they were able to securely query over 100,000 patient samples across five continents without a single byte of raw data leaving its original jurisdiction.
The result? They identified three high-confidence targets for a rare neurological condition and moved to lead optimization in under six months, cutting their traditional findy timeline by over 50%. This was achieved by using Lifebit’s automated pipelines to harmonize disparate electronic health records (EHR) with whole-genome sequencing data, providing a holistic view of the disease phenotype that was previously impossible to see.
Federated AI: Unlocking Global Biomedical Data Without Compromising Privacy
The future of drug development is global, but data privacy laws like GDPR and HIPAA often make sharing data impossible. Lifebit’s federated approach solves this. Instead of moving sensitive data to the AI, we bring the AI to the data. This “compute-to-data” paradigm allows for secure, privacy-preserving analytics that enable cross-border collaborations between governments, biobanks, and biopharma giants.
This is particularly critical for rare disease research, where no single hospital or even country has enough patients to provide a statistically significant sample size. By federating access to multiple biobanks, Lifebit allows AI models to train on a diverse, global population, leading to more robust and generalizable findings.
Multi-Omics at Scale: From Genomics to Real-World Evidence
To truly understand disease, you need more than just DNA. You need transcriptomics (how genes are expressed), proteomics (the proteins actually present), and real-world evidence (RWE) (how the patient actually lives and responds to treatment). Lifebit’s R.E.A.L. (Real-time Evidence & Analytics Layer) allows researchers to blend these layers seamlessly.
This holistic view is exactly what leading research teams use to decode aging pathways or metabolic diseases. By integrating these massive datasets, we help our partners design trials with higher success rates. For example, by identifying a specific proteomic biomarker in a sub-population of patients, a pharma company can narrow its clinical trial recruitment to only those most likely to respond, significantly increasing the probability of trial success and reducing the “noise” in clinical data.
Data Quality and Regulatory Readiness: How Lifebit Delivers
AI is only as good as the data it consumes. As industry leaders have noted, robust data and validation are key to drug approvals. Lifebit ensures this through automated data validation and full audit trails. Every action taken within our platform—from data cleaning to model training—is logged and reproducible.
Our platform is built to meet FDA and EMA guidance on AI transparency and “Explainable AI” (XAI). This ensures that when your AI-designed molecule hits the clinic, the data backing it is unimpeachable. We provide the “provenance” of the data, showing exactly how a specific insight was derived, which is essential for regulatory submissions where “black box” algorithms are increasingly scrutinized.
Strategic Partnerships: How Leading Pharma Trusts Lifebit
The “Pharma AI Readiness Index” shows that leaders like top-10 global pharma organizations are winning because they prioritize external activity. They don’t just build internal tools; they form strategic alliances that allow them to tap into the best data and the best algorithms simultaneously. The era of the “closed lab” is over; the era of the “innovation ecosystem” has begun.
Lifebit is proud to be the infrastructure partner for many of these leaders. By providing a Trusted Data Lakehouse (TDL) and federated governance, we allow global pharmaceutical companies to share data securely with academic partners and specialized AI teams. These collaborations have already fueled breakthroughs in oncology and immunology, proving that when the right data meets the right AI, the results are life-changing.
Furthermore, these partnerships extend to public-private initiatives. Lifebit powers environments where pharmaceutical companies can collaborate with national health services (like the NHS in the UK) to gain insights from large-scale population data while maintaining the highest standards of public trust and data security. This synergy between public health data and private R&D is the engine of the next generation of precision medicine.
Frequently Asked Questions about AI in Pharma
What is the success rate of AI-designed drugs?
While no AI-designed drug has reached full FDA approval yet, the early data is staggering. AI-nominated compounds have shown Phase I success rates of 80-90%, nearly double the industry average of 40-65%. This suggests that AI is much better at picking “winners” during the findy phase by predicting toxicity and bioavailability issues before a single human is ever dosed. However, the real test will be Phase II and III, where efficacy in large, diverse populations is proven.
How much time does AI save in drug findy?
Leading AI-first drug development programs report that AI can compress the findy-to-preclinical phase from the traditional 5 years down to 18-24 months. Some design cycles are now 70% faster, requiring 10 times fewer compounds to be physically synthesized in the lab. This speed doesn’t just save money; it brings life-saving treatments to patients years earlier than previously possible.
How does Lifebit lead in AI drug development?
Lifebit leads by solving the “data access” problem. While other companies focus on the algorithms, we provide the secure, federated environment where those algorithms can actually work. Our platform is the only one that enables real-time, compliant access to global multi-omic data at scale. We act as the “operating system” for AI drug discovery, ensuring that the data is clean, the environment is secure, and the results are reproducible.
Can AI help with “undruggable” targets?
Yes. Many proteins lack a deep binding pocket where a traditional drug can sit. AI models can simulate the “breathing” of proteins to find temporary openings (cryptic pockets) or design “molecular glues” that bring two proteins together to trigger a therapeutic effect. This opens up a massive range of diseases that were previously considered untreatable.
Is AI replacing medicinal chemists?
No. AI is a powerful tool that augments the expertise of medicinal chemists. It handles the “brute force” work of screening billions of molecules and predicting properties, allowing chemists to focus on high-level strategy, creative problem-solving, and the complex nuances of human biology that models cannot yet fully capture. The most successful companies are those that foster a “human-in-the-loop” approach.
What are the regulatory hurdles for AI-designed drugs?
Regulators like the FDA and EMA are concerned with the “explainability” of AI. They need to know why an AI chose a specific molecule. Companies leading the way are using “Explainable AI” and maintaining rigorous data provenance—tracking every step of the data’s journey—to satisfy these requirements. Lifebit’s platform is specifically designed to provide this level of transparency and auditability.
Conclusion: Don’t Let Legacy Data Hold You Back
The question is no longer if AI will change drug development, but which companies will survive the transition. The pharmaceutical landscape is shifting from a model of high-volume, high-failure experimentation to one of high-precision, data-driven engineering. Leading the way requires more than just a smart algorithm; it requires a robust, secure, and federated data strategy that can tap into the world’s most valuable biomedical insights.
As we look toward 2030, the companies that will dominate the market are those that have successfully integrated AI across their entire value chain—from the earliest stages of target discovery to the optimization of clinical trials and the monitoring of real-world patient outcomes. This integration is only possible when data is treated as a strategic asset rather than a siloed liability.
At Lifebit, we are committed to helping you turn your data into your greatest competitive advantage. Whether you are looking to accelerate target identification, optimize your clinical trials, or secure global research collaborations, our platform is built to get you there faster. The race to the next breakthrough is on. Learn how Lifebit accelerates your drug pipeline today and join the ranks of the companies leading the way in using AI in drug development.

