Ultimate Checklist for Top Clinical Data Integration Providers

Stop Wasting 80% of Your Research Time: The 2025 Guide to Clinical Data Integration
What are the top clinical data integration platform providers for pharmaceutical research? Leading platforms include federated AI systems like Lifebit (enabling secure analysis across 187M+ patient records without data movement), Clinical Data Workbenches (unifying EDC, labs, and wearables into single-source repositories), bioprocess integration engines (connecting bioreactor data with MES systems for real-time manufacturing insights), and enterprise data platforms (providing lakehouse architecture for multi-modal clinical datasets). These solutions address the core challenge facing pharmaceutical organizations: 65% of clinical trials now pull data from six or more external sources, yet 30% still face prolonged timelines due to fragmented systems and manual data wrangling.
Quick comparison of top provider categories:
| Provider Type | Primary Use Case | Key Strength |
|---|---|---|
| Federated AI Platforms (e.g., Lifebit) | Multi-site genomics, real-world evidence | Analyze data in situ without physical movement |
| Clinical Data Workbenches | Trial conduct, EDC integration | Automated SDTM change and query management |
| Bioprocess Engines | Manufacturing quality control | Real-time bioreactor monitoring with 21 CFR Part 11 compliance |
| Data Lakehouse Platforms | Enterprise analytics, imaging data | Scalable architecture for petabyte-scale multi-omics |
The stakes are high. A typical Phase III trial now generates 3.6 million data points-three times more than 15 years ago-while delays cost up to $8 million per day. Yet most organizations still rely on hybrid digital-paper systems that turn what should be hours of analysis into months of manual cleanup. The research is clear: 78% of healthcare providers using proper integration standards experience faster care coordination and better patient outcomes, and organizations leveraging AI-driven insights report a 40% increase in study report accuracy.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over a decade building federated genomics infrastructure that powers secure analysis across hundreds of millions of patient records-and I’ve seen what are the top clinical data integration platform providers for pharmaceutical research doing to transform an industry drowning in data silos. Before founding Lifebit, I contributed to Nextflow and led computational biology research at the Centre for Genomic Regulation, giving me a front-row seat to the evolution from fragmented best-of-breed tools to unified intelligence platforms.
Quick What are the top clinical data integration platform providers for pharmaceutical research? definitions:
Why 70% of Your Clinical Data Is Useless-And How to Fix It

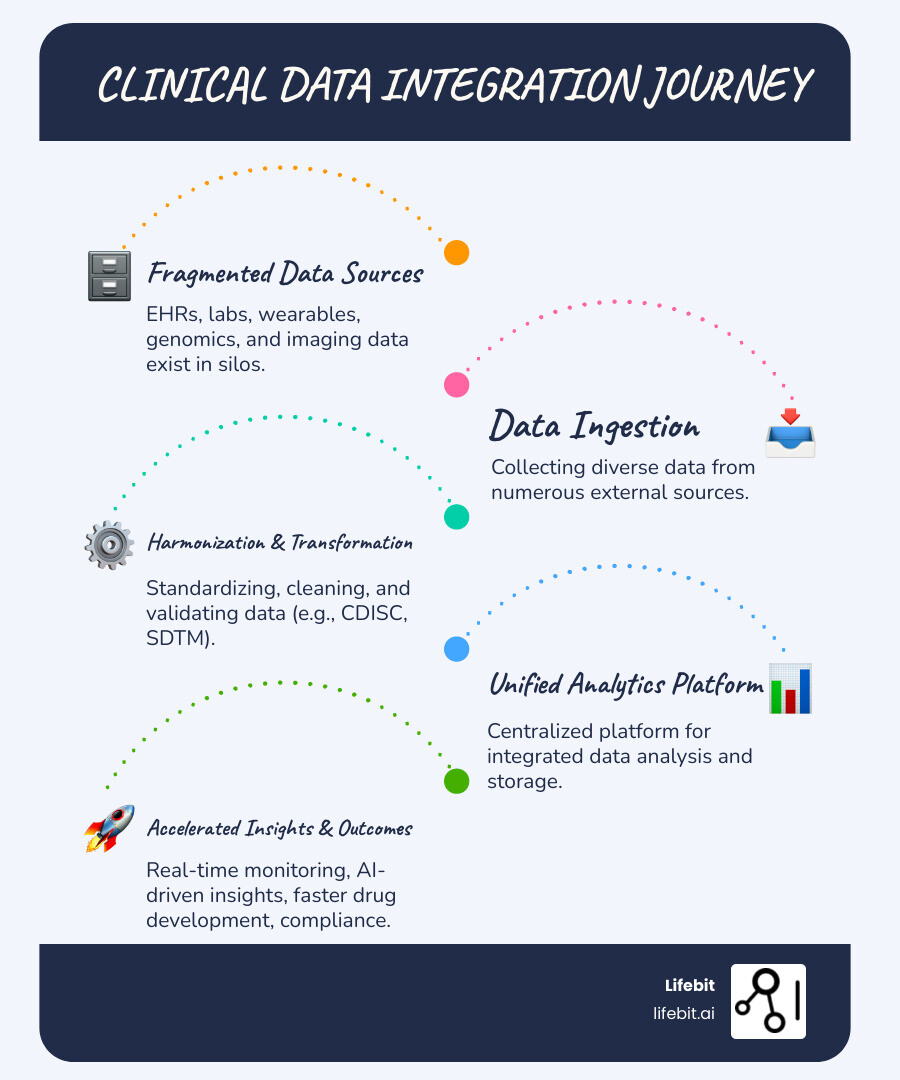
In the current landscape of drug discovery, we are swimming in data but starving for insights. A recent survey of clinical trial sponsors revealed that 65% of respondents use six or more external data sources within their studies, and nearly 29% use more than 10. Despite this abundance, the path from raw data to a “database lock” is riddled with roadblocks. This phenomenon is often referred to as the “Data Tax”—the hidden cost of managing complexity that drains resources away from actual scientific innovation.
The primary culprit? Fragmentation. When data sits in siloed lab systems, wearable device clouds, and disparate Electronic Data Capture (EDC) tools, researchers spend up to 80% of their time on manual data wrangling. This isn’t just a minor inconvenience; it is a systemic failure of legacy infrastructure. According to Scientific research on clinical data management challenges, this lack of integration leads to:
- Prolonged Timelines: 30% of study teams report being unable to meet target timeframes from the last patient’s visit to final tables, listings, and figures (TLFs). Every month of delay in a Phase III trial can represent tens of millions in lost potential revenue.
- Quality Erosion: 30% encounter significant problems with data quality, often due to manual entry errors or “dirty” data from third-party sources. When data is moved manually between systems, the risk of transcription errors increases exponentially, leading to “data debt” that must be paid during the regulatory audit phase.
- Software Rigidity: 19% of specialists face software that simply isn’t flexible enough to integrate the necessary data types, such as high-resolution imaging, digital biomarkers from wearables, or multi-omics. Legacy systems were built for a world of paper forms, not the petabyte-scale reality of modern precision medicine.
- The “Data Janitor” Syndrome: Highly paid bioinformaticians and clinical data scientists spend the majority of their workweek performing ETL (Extract, Transform, Load) tasks rather than building predictive models or identifying safety signals. This leads to high turnover and burnout in critical R&D roles.
When we talk about what are the top clinical data integration platform providers for pharmaceutical research, we are really talking about the tools that stop this hemorrhage of time and money. Every day a drug is delayed from reaching the market costs between $600,000 and $8 million. For researchers, the pain is real: they are “blind and deaf in the middle of a freeway” without a unified view of the patient. To solve this, the industry is moving toward “Data Fabric” architectures that weave together disparate sources into a cohesive, searchable, and actionable whole.
What are the top clinical data integration platform providers for pharmaceutical research? The Shift to Unified Intelligence
The market has shifted from “best-of-breed” point solutions to unified platforms. The goal is no longer just to store data, but to create a Data Intelligence Platform that automates the flow from ingestion to insight. This evolution is driven by the need for “Real-Time Data Flow,” where data is validated the moment it is captured, rather than months later during a reconciliation phase.
Federated AI Platforms for Global Biomedical Data
At Lifebit, we believe the old model of “moving data to the code” is broken. It is too slow, too expensive, and creates massive security risks. Our next-generation federated AI platform allows researchers to bring their analysis to the data, wherever it resides globally. This is particularly critical for genomic data, which is often subject to strict national sovereignty laws.
By using a Trusted Research Environment (TRE) and a Trusted Data Lakehouse (TDL), we enable secure, real-time access to multi-omic and clinical data across five continents. Our R.E.A.L. (Real-time Evidence & Analytics Layer) provides the AI-driven safety surveillance and pharmacovigilance insights that modern biopharma needs. This federated approach is crucial for complying with strict local data residency laws in regions like Europe (GDPR) and Singapore while still allowing for large-scale, global collaboration. Instead of waiting months for data transfer agreements and physical hard drive shipments, researchers can run queries across global cohorts in minutes.
What are the top clinical data integration platform providers for pharmaceutical research workbenches?
For clinical trial operations, the “Clinical Data Workbench” has emerged as the gold standard. These platforms act as a single source of truth, pulling in the 70% of data that now comes from external sources like central labs, ePRO (electronic Patient-Reported Outcomes), and wearable sensors.
Key players in this space, such as Veeva Systems and Medidata (Dassault Systèmes), focus on:
- Automated Harmonization: Mapping diverse data into standard models like CDISC SDTM or OMOP without manual coding. This allows for “push-button” regulatory submissions.
- Query Management: Tracking data discrepancies across refreshed datasets to avoid redundant reviews. If a lab value changes in the source system, the workbench automatically flags the discrepancy for the clinical monitor.
- Patient Profiles: Providing a holistic view of individual participants to speed up medical and safety reviews. This includes visualizing trends in vital signs alongside adverse event reports to identify correlations that might otherwise be missed.
Top-tier platforms have dominated this sector by offering out-of-the-box integrations with major EDC systems. They reduce the time from data ingestion to “clean” status from months to days, which is essential for agile trial management and adaptive trial designs where the study protocol might change based on interim data analysis.
Enterprise Data Lakehouses and Cloud Infrastructure
Beyond specialized clinical tools, general-purpose data giants like Databricks and Snowflake have made significant inroads into pharmaceutical research. These providers offer the “Lakehouse” architecture—a hybrid that combines the cost-effectiveness of a data lake with the performance and ACID compliance of a data warehouse.
For pharma, this means being able to store massive amounts of unstructured data (like MRI scans or pathology slides) alongside structured clinical trial data. These platforms provide the computational muscle needed for large-scale machine learning, such as training Large Language Models (LLMs) on internal research papers to identify new drug targets. When integrated with specialized life sciences layers, these lakehouses become the backbone of a company’s entire R&D strategy.
Bioprocess and Manufacturing Integration Engines
Data integration doesn’t stop at the clinical trial; it extends into the bioreactor. Pharmaceutical manufacturers face a “paradox of data overabundance” in bioprocessing. Modern platforms now offer AI-powered unification for manufacturing teams, ensuring that the transition from the lab to the factory floor is data-driven.
These engines are built for 21 CFR Part 11 compliance, ensuring full audit trails and data integrity. They use soft sensors—mathematical models that estimate variables that are difficult to measure directly—to monitor Critical Process Parameters (CPPs) in real-time. This can reduce batch failures by 25%, saving millions in wasted materials. By integrating Electronic Batch Records (EBR) with analytical instrument data, these platforms ensure that “tech transfer” between R&D and manufacturing is seamless, verifiable, and ready for FDA inspection.
5 Features That Cut Clinical Data Review Time by 80%
When evaluating what are the top clinical data integration platform providers for pharmaceutical research, you must look beyond the marketing gloss. A platform is only as good as its ability to handle the “messy” reality of biomedical data. The following five features are non-negotiable for any organization looking to scale their research capabilities:
-
AI/ML-Driven Automation & Semantic Mapping: AI can now shorten development timelines by an average of six months. Look for platforms that use agentic AI for semantic mapping—automatically recognizing that “Heart Attack” in one system is “Myocardial Infarction” in another. This eliminates the need for manual “data cleaning” and ensures that datasets from different hospitals or countries can be pooled for analysis without losing context. Advanced platforms now use Natural Language Processing (NLP) to extract data from unstructured clinical notes, turning narrative text into structured data points.
-
Adherence to FAIR Principles: Data must be Findable, Accessible, Interoperable, and Reusable. Scientific research on FAIR data principles shows that this framework is the only way to ensure long-term value from research investments. A platform that locks your data into a proprietary format is a liability. Top providers use open standards and provide robust APIs (Application Programming Interfaces) to ensure that data can be moved and reused across different research projects over decades.
-
Robust Security & Federated Governance: With the rise of GDPR, HIPAA, and evolving FDA guidelines, a platform must provide “privacy by design.” This includes anonymization, pseudonymization, and secure Trusted Research Environments (TREs). The most advanced platforms use Differential Privacy and Homomorphic Encryption to allow analysis on encrypted data, ensuring that even the platform provider cannot see sensitive patient information. This level of security is essential for building trust with patients and healthcare providers.
-
Interoperability Standards (FHIR, HL7, CDISC): Ensure the platform natively supports FHIR (Fast Healthcare Interoperability Resources), HL7, and CDISC. A platform that requires custom code for every new integration is just another silo in the making. Native support for these standards allows for “plug-and-play” connectivity with Electronic Health Records (EHRs) and laboratory information management systems (LIMS), drastically reducing the time required for site startup in clinical trials.
-
Real-Time Pipelines & Streaming Analytics: In an era of decentralized trials and wearables, “batch processing” is dead. You need real-time data streaming to detect safety signals the moment they occur. If a patient’s wearable detects a cardiac anomaly, that data should flow into the integration platform and trigger an alert for the medical monitor within seconds, not weeks. This capability is the foundation of modern pharmacovigilance and patient safety.
Cut 6 Months Off Your Drug Development Timeline with Integrated Data
The ROI of clinical data integration isn’t just a theoretical exercise—it’s measured in lives saved and billions of dollars in efficiency gains. By moving away from manual validation and embracing automated, unified platforms, biopharma companies are seeing transformative results across the entire drug development lifecycle.
- 97% Reduction in Coding Time: AI-driven tools can take tasks that used to take weeks—such as mapping clinical trial data to the SDTM standard—and finish them in hours. This allows clinical programmers to focus on high-value analysis rather than repetitive data entry.
- 40% Increase in Accuracy: Automated cleaning and outlier detection significantly reduce the risk of regulatory “Warning Letters” due to data integrity issues. By removing the human element from data transformation, organizations ensure a “gold standard” dataset that stands up to the most rigorous regulatory scrutiny.
- $25,000 Savings Per Patient: By using FDA-ready data and reducing rework, organizations can drastically lower the cost of participant enrollment and management. When data flows seamlessly from the site to the sponsor, the need for expensive On-Site Monitoring (OSM) is reduced, replaced by more efficient Risk-Based Monitoring (RBM).
- Accelerated Patient Recruitment: Integrated platforms allow researchers to query vast networks of Real-World Data (RWD) to identify patient cohorts that meet specific inclusion/exclusion criteria. This can reduce recruitment timelines by 30-50%, which is often the single biggest bottleneck in clinical research.
Furthermore, the rise of Decentralized Clinical Trials (DCT)—a market projected to reach $16.29 billion by 2027—is only possible through these integration platforms. With eConsent usage increasing by 460%, the ability to harmonize data from a patient’s smartphone with their clinical site records is the new baseline for success. Companies that fail to integrate these disparate streams will find themselves unable to compete in a world where patient-centricity is the primary driver of trial participation. The ultimate goal is a “Continuous Evidence” model, where data from clinical trials, real-world use, and post-market surveillance flow into a single intelligence loop, informing the next generation of therapies.
Frequently Asked Questions: Solving the Clinical Data Integration Crisis
What are the top clinical data integration platform providers for pharmaceutical research?
The top providers are those that offer a unified architecture capable of aggregating data from EDC, labs, wearables, and genomics. Leading names include Lifebit for federated biomedical data and genomics, Veeva and Medidata for clinical trial workbenches, and Databricks or Snowflake for enterprise-scale data lakehouses. The “best” provider depends on your specific needs: federated access for global data, or deep EDC integration for trial operations.
How do these platforms ensure HIPAA and GDPR compliance?
Top-tier platforms use Trusted Research Environments (TREs) and federated governance. This ensures that sensitive patient data remains in its original jurisdiction (staying behind the provider’s firewall) while only the “answers” or insights are shared. This approach provides a robust audit trail, manages consent at a granular level, and ensures that data is anonymized according to the highest regulatory standards for FDA and EMA submissions. They also employ “Zero Trust” security architectures, where every access request is strictly verified.
What are the top clinical data integration platform providers for pharmaceutical research use cases involving AI?
Platforms like Lifebit are leaders in AI integration. They use machine learning to automate the ingestion and cleaning of multi-modal data (combining imaging, genomics, and EHRs). By leveraging agentic AI for semantic mapping and predictive analytics, these providers help researchers identify patient cohorts 50% faster and shorten the overall drug development cycle by an average of six months per asset. Other providers are integrating LLMs to help researchers “talk to their data” using natural language queries.
Can these platforms handle data from wearable devices and IoT?
Yes, modern integration platforms are designed to handle high-frequency, high-volume data from wearables. They use specialized “ingestion engines” that can process millions of data points per second, filtering out noise and identifying clinically relevant signals. This is essential for digital biomarker development, where continuous monitoring of a patient’s gait, heart rate, or sleep patterns can provide a more accurate picture of drug efficacy than occasional clinic visits.
What is the difference between a Data Lake and a Data Lakehouse in pharma?
A Data Lake is a vast repository of raw data in its native format. While useful for storage, it often becomes a “Data Swamp” where information is hard to find and lacks quality controls. A Data Lakehouse (like those provided by Databricks or Snowflake) adds a layer of structure and governance on top of the lake. It allows for high-performance querying and ensures data integrity, making it suitable for the rigorous requirements of pharmaceutical R&D and regulatory reporting.
Stop Wrangling Data and Start Finding Cures
The future of pharmaceutical research is not just “digital”-it is integrated. As we move toward 2026, the ability to bridge the gap between clinical trials, real-world evidence, and multi-omics will define the winners in the biopharma race.
By choosing a platform that prioritizes federated access, AI-driven automation, and strict regulatory compliance, you aren’t just buying software; you’re building a foundation for scalable research and faster medical breakthroughs. If you’re ready to stop wrangling data and start finding cures, learn more about Lifebit’s platform and how we can help you open up the power of global biomedical data.

