The Robot Will See You Now for Your Rare Condition

Why 80% of Rare Diseases Need AI Right Now
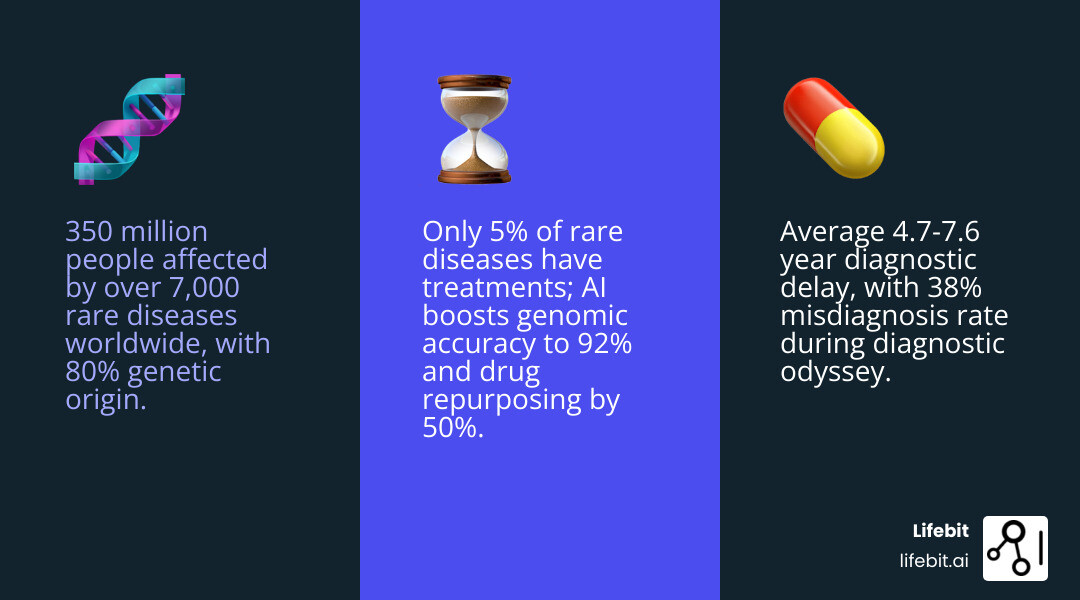
Rare disease AI is changing how doctors diagnose and treat over 7,000 rare conditions affecting 350 million people worldwide. Here’s what you need to know:
| Challenge | AI Solution | Impact |
|---|---|---|
| 4.7-year average diagnostic delay | Machine learning analyzes phenotypes and genomics | ChatGPT identified 13.3% of cases vs. 5.6% clinical review |
| Only 5% of rare diseases have treatments | AI drug repurposing and discovery | 50% higher accuracy in candidate identification |
| 38% of patients get misdiagnosed | Facial recognition and genome analysis | 92% accuracy ranking correct genes in top two |
| $219,000 average annual orphan drug cost | Faster clinical trials with synthetic controls | 87.6% accuracy screening trial eligibility |
Nearly 80% of rare diseases are genetic, yet the average patient waits 7.6 years from symptom onset to diagnosis. That’s 7.6 years of wrong treatments, mounting medical bills, and deteriorating health.
The numbers paint a grim picture: only 30% of rare disease patients receive an accurate diagnosis from standard exome sequencing. Meanwhile, 38% receive at least one misdiagnosis during their diagnostic odyssey. For pediatric patients, symptoms often appear at a median age of just 7.6 months—but answers come years later, if they come at all.
Traditional drug discovery isn’t solving the problem either. With a 5% success rate and a focus on blockbuster drugs for large patient populations, pharmaceutical companies have historically ignored rare diseases. The result? Effective treatments exist for only 5% of the 7,000+ known rare diseases.
Artificial Intelligence is changing this equation. AI systems now rank the correct disease-causing gene within the top two candidates in 92% of cases. Large language models like ChatGPT diagnose rare diseases at a 13.3% rate—more than double the 5.6% historical clinical review rate—at a cost of just $0.03 and five seconds per case.
Deep learning models analyze facial images to recognize over 200 syndromes with 91% top-10 accuracy. Graph neural networks identify drug repurposing candidates with 50% higher accuracy than conventional approaches. And AI-powered clinical trial screening achieves 87.6% accuracy using electronic health record data, dramatically speeding up patient recruitment.
The technology addresses rare diseases’ biggest challenge: data scarcity. Through transfer learning, federated analysis, and few-shot learning, AI models trained on common diseases adapt to rare conditions with minimal patient data. This approach unlocks insights from the 98% of the genome that standard exome sequencing ignores—including noncoding promoter regions responsible for up to 6% of rare disease genetic causes.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built a pioneering federated genomics platform that enables secure, compliant analysis of biomedical data for rare disease AI applications across pharmaceutical and public sector institutions. With over 15 years in computational biology, AI, and precision medicine, I’ve seen how rare disease AI transforms patient outcomes when researchers can access diverse datasets without compromising privacy.
Rare disease AI further reading:
- ai-driven drug discovery
- List software providers that use AI for drug discovery.
- Which companies are leading the way in using AI in drug development?
The $219,000 Problem: Why Rare Disease Diagnosis Fails Patients
The financial and emotional toll of a rare disease is staggering. In the United States, the average annual treatment cost for orphan drugs reaches approximately USD 219,000. But before a patient even reaches the pharmacy counter, they must survive the “diagnostic odyssey.”
This odyssey is not merely a medical delay; it is an economic and psychological crisis. According to the EveryLife Foundation for Rare Diseases, the total economic impact of rare diseases in the U.S. was estimated at nearly $966 billion in a single year. Of this, $418 billion represents direct medical costs, while a staggering $548 billion accounts for non-medical costs and lost productivity. Families often bear the brunt of these expenses, including home modifications, specialized transportation, and the loss of income as parents or spouses become full-time caregivers.
For many, this journey is a maze of dead ends. Statistics show that 28% of rare disease patients wait seven years or more for an accurate diagnosis. During this time, approximately 38% of patients receive at least one misdiagnosis, leading to inappropriate treatments that can sometimes cause more harm than the disease itself. Patients often visit an average of eight different physicians—including primary care doctors and multiple specialists—before receiving a correct diagnosis. This “doctor-shopping” is rarely by choice; it is a desperate search for answers in a system not designed for the exceptional.
The delay is particularly heartbreaking for families with young children. The median age at symptom onset is often just 7.6 months, yet the median length of the diagnostic odyssey is 7.6 years. This means many children spend their entire early childhood without a name for their condition, missing critical windows for early intervention. Scientific research on AI’s impact in rare disease diagnosis highlights that these delays aren’t just frustrating—they are medically dangerous. For instance, in metabolic rare diseases like Pompe disease or Spinal Muscular Atrophy (SMA), a delay of just a few months can mean the difference between a normal life and permanent neurological impairment or loss of motor function.
By integrating rare disease genomics, we can start to peel back the layers of these complex conditions. However, the sheer volume of data is too much for any human doctor to process alone. A single whole-genome sequence generates roughly 100 gigabytes of raw data, containing millions of variants. With over 7,000 known rare diseases, no clinician can be an expert in everything. This is where rare disease AI steps in to act as a tireless, ultra-intelligent assistant, capable of cross-referencing a patient’s unique genetic markers against the entirety of published medical literature—over 30 million papers—in seconds.
Rare Disease AI: Instantly Spot 7,000+ Conditions with Smarter Tools
We believe that no patient should have to wait a decade for an answer. Modern rare disease AI tools, specifically Machine Learning (ML) and Deep Learning (DL), are designed to process the “unstructured” data that humans often miss—like the nuanced notes in an Electronic Health Record (EHR) or subtle patterns in facial features.
One of the most powerful applications is phenotype analysis. Scientific research on phenotype analysis development shows how standardized vocabularies, like the Human Phenotype Ontology (HPO)—which includes over 13,000 terms—allow AI to match a patient’s symptoms to a specific genetic disorder. AI systems use Natural Language Processing (NLP) to scan through years of physician notes, extracting these HPO terms automatically to build a comprehensive “digital patient profile.” This is crucial because rare disease symptoms are often “noisy” and spread across different organ systems, making it difficult for a human specialist to see the overarching pattern.
By leveraging high-quality patient registries, AI can compare one patient’s rare symptoms against thousands of others globally, finding “look-alike” cases that would otherwise remain hidden in local hospital silos. This is particularly effective for ultra-rare diseases where only a handful of cases exist worldwide. For example, if a child in Tokyo and a child in London share a unique combination of cardiac anomalies and specific skeletal growth patterns, AI can flag this connection instantly, even if the individual doctors have never seen the condition before.
AI Tools for Genomic and Phenotype Screening
The “first generation” of AI focused on simple pattern matching. Today, we are seeing “second-generation” systems that are dynamic and patient-centered. For example:
- DeepGestalt and Computer Vision: A deep learning model trained on over 17,000 facial images that can recognize more than 200 syndromes with a 91% top-10 accuracy. It identifies subtle dysmorphic features—such as the specific slant of the eyes, the shape of the philtrum, or the positioning of the ears—that are characteristic of conditions like Cornelia de Lange syndrome or Angelman syndrome. These features are often so subtle that they are missed by non-geneticists.
- Whole Genome Interpretation: Systems that can rank the correct disease-causing gene within the top two candidates in 92% of cases. These tools use Bayesian networks to weigh the pathogenicity of variants against the patient’s clinical presentation, filtering out the “background noise” of benign genetic variation that makes manual interpretation so time-consuming.
- Noncoding Variant Analysis: Tools like PromoterAI now decipher pathogenic variants in the 98% of our genome that was previously considered “junk DNA.” By identifying mutations in regulatory regions that control gene expression, these tools are potentially doubling the diagnostic yield for patients who previously had “negative” exome results.
Large Language Models: Fast, Affordable Rare Disease Diagnosis
Large Language Models (LLMs) like ChatGPT and Llama are proving to be surprisingly effective diagnostic partners. In studies conducted with the Undiagnosed Diseases Network (UDN), LLMs achieved diagnostic rates of 13.3% (ChatGPT) and 10.0% (Llama), compared to a historical clinical review rate of just 5.6%.
The efficiency is even more shocking: ChatGPT processed these complex cases for just $0.03 per case in five seconds. While they aren’t replacing doctors, they provide “helpful” diagnostic suggestions in nearly a quarter of cases, acting as a high-speed second opinion for exhausted clinical teams. These models are particularly adept at “connecting the dots” between seemingly unrelated symptoms—such as a combination of hearing loss, kidney issues, and specific skin pigmentations—that are hallmarks of multisystemic disorders like Alport syndrome or Waardenburg syndrome.
Accelerating Treatment: How AI Fixes the 95% Drug Failure Rate
Finding a diagnosis is only half the battle. For the 95% of rare diseases that currently have no approved treatment, we need a faster way to develop drugs. Traditional drug discovery is slow, expensive, and has a 95% failure rate. The “Valley of Death” in drug development—the gap between laboratory discovery and clinical application—is particularly wide for rare diseases due to small patient populations and limited commercial incentives. It typically takes 12-15 years and over $2.6 billion to bring a single new drug to market; for a disease affecting only 1,000 people, this math simply doesn’t work.
Scientific research on AI in drug discovery suggests that AI can flip this script through drug repurposing. Instead of spending a decade developing a new molecule, we can use AI to scan existing, FDA-approved drugs to see if they can treat a rare condition. This approach leverages Graph Neural Networks (GNNs) to map the complex interactions between drugs, proteins, and diseases. By treating the human body as a massive “knowledge graph,” AI can predict how a drug designed for one condition might interact with the biological pathways of another.
A perfect example is the discovery of treatments for Castleman’s disease. AI platforms have successfully identified existing medications, like adalimumab (originally for rheumatoid arthritis), for patients who were previously out of options. By using zero-shot learning—where an AI can predict a treatment for a disease it has never seen before—tools like TxGNN are demonstrating 50% higher accuracy in identifying therapeutic candidates than conventional methods. You can learn more about drug repurposing for rare conditions and how these “old” drugs are saving “new” lives.
AI in Clinical Trials and Synthetic Controls
Clinical trials for rare diseases are notoriously difficult because there are so few patients to enroll. Rare disease AI solves this by:
- Automated Screening and Patient Stratification: AI systems can evaluate clinical trial eligibility using EHR data with 87.6% accuracy. Beyond just finding patients, AI can stratify them based on their likely rate of disease progression. This ensures that trial cohorts are balanced, preventing a scenario where a drug appears ineffective simply because the enrolled patients had a slower-progressing form of the disease.
- Synthetic Control Arms (SCAs): Instead of giving half of a small patient group a placebo—which is often ethically problematic in terminal rare diseases—researchers can use AI to create “synthetic controls” based on real-world data (RWD). By using historical data from previous trials, natural history studies, and electronic health records, AI can simulate how a control group would respond. This allows every enrolled patient to receive the experimental treatment, making trials more attractive to participants and significantly faster to complete.
- Registry Integration and Real-World Evidence: By using clinical registry solutions, we can track long-term treatment responses in real-time. AI models can predict which patients are likely to respond to a specific therapy, enabling a “precision trial” approach. This is particularly vital for regulatory bodies like the FDA and EMA, which are increasingly accepting AI-generated real-world evidence to support orphan drug approvals.
Solving the Data Scarcity Trap: Transfer Learning and Federated Access
The biggest hurdle for rare disease AI is the lack of data. How do you train a “big data” model on a disease that only affects 50 people? Traditional AI requires thousands of examples to learn a pattern, but in the rare disease world, data is the most precious and limited resource. Furthermore, this data is often locked in “silos”—individual hospitals or national health systems that cannot share data due to strict privacy laws like GDPR or HIPAA.
We use three primary strategies to overcome this “scarcity trap”:
- Transfer Learning: We train an AI model on a common disease (like diabetes or breast cancer) where data is plentiful, and then “fine-tune” it on the small rare disease dataset. Scientific research on transfer learning shows this helps the model “understand” basic biological principles—such as how proteins fold or how metabolic pathways interact—before it ever sees a rare case. It’s like teaching a student general medicine before they specialize in a rare sub-field.
- Data Augmentation & Generative AI: We can use AI to create “synthetic patients”—digital avatars that mimic the biological characteristics of real rare disease patients. These Generative Adversarial Networks (GANs) can expand a dataset of 10 patients into a statistically significant cohort of 1,000. This allows for more robust model training without compromising the privacy of the original individuals, as the synthetic data contains no real patient identifiers.
- Federated Learning: This is our specialty at Lifebit. Instead of moving sensitive patient data to the AI (which creates massive privacy risks), we move the AI to the data. This allows us to analyze rare disease registries across different countries—from Canada to the UK to Israel—without the data ever leaving its secure home. In a federated setup, the model is trained locally at each site, and only the “learned weights” (mathematical insights) are sent to a central server to be aggregated. This “decentralized” approach is essential for rare diseases, as no single country has enough data to train an accurate model on its own. By connecting these global silos, we create a “virtual” massive dataset that respects national data sovereignty and patient confidentiality.
The Future of Precision Medicine: Ethics, Interpretability, and Global Scale
As we look toward 2026 and beyond, the focus is shifting from “Can AI do it?” to “Can we trust it?” and “Is it fair?” The integration of AI into clinical workflows requires more than just accuracy; it requires accountability and transparency.
Model interpretability is key. Doctors cannot and should not make life-altering decisions based on a “black box.” They need to know why an AI suggested a specific diagnosis. Using techniques like SHAP (SHapley Additive exPlanations) and LIME, we can make the AI transparent, showing exactly which symptoms, genetic markers, or lab values led to a conclusion. For example, an AI might highlight that its diagnosis of Fabry disease was 80% driven by a specific combination of “burning pain in hands” and “decreased sweating” found in the EHR notes, combined with a specific variant in the GLA gene. This builds the necessary trust between the clinician, the AI, and the patient.
Scientific research on rare disease monitoring emphasizes the need for “human-in-the-loop” frameworks. AI shouldn’t be the final judge; it should be the ultimate tool for the clinician. Furthermore, we must address the diversity gap in genomic data. Currently, over 80% of genomic data used in research comes from populations of European descent. This creates a “genomic bias” where AI tools may be less accurate for patients of African, Asian, or Hispanic descent. For rare disease AI to be truly global, we must use federated platforms to include diverse datasets from the Global South, ensuring that diagnostic tools work for everyone, regardless of their ancestry.
The future also holds the promise of pre-symptomatic intervention. Imagine an AI that scans newborn genomic data and identifies a rare condition years before symptoms appear. This would allow for personalized RNA-based drugs or gene therapies to be developed and administered before the child ever gets sick. We are also seeing the rise of “Digital Twins”—virtual models of a patient’s unique biology that allow doctors to test different treatments in a simulation before giving them to the patient. By simulating how a specific drug will interact with a patient’s unique genetic makeup, we can avoid the “trial and error” approach that currently defines rare disease care. This isn’t science fiction—it’s the direction of modern precision medicine, where the goal is to move from reactive treatment to proactive prevention.
Frequently Asked Questions about Rare Disease AI
How fast can AI diagnose a rare disease?
While a traditional diagnostic odyssey takes an average of 4.7 to 7.6 years, AI tools like LLMs can provide diagnostic suggestions in seconds. In genomic analysis, AI-powered interpretation systems can rank the correct gene in minutes, potentially reducing the wait from years to days.
Can AI help find treatments for untreatable diseases?
Yes. AI excels at drug repurposing, identifying existing medications that can be used for new purposes. AI models have already identified candidates for rare inflammatory myopathies and Castleman’s disease, often with 50% better accuracy than traditional methods.
Is patient data secure with AI in rare disease care?
Security is our top priority. By using federated AI platforms, we ensure that data remains behind the hospital’s or government’s firewall. Researchers can gain insights and train models without ever seeing or moving the raw, identifiable patient data, ensuring full HIPAA and GDPR compliance.
Conclusion
The “diagnostic odyssey” has been a dark chapter for millions of families, but rare disease AI is finally turning the lights on. From facial recognition software that spots genetic syndromes to federated learning that connects global registries, technology is closing the gap that traditional medicine left behind.
At Lifebit, we are proud to provide the next-generation federated AI platform that makes this possible. Our Trusted Research Environment (TRE) and R.E.A.L. analytics layer enable secure, real-time access to the multi-omic data needed to solve the world’s rarest medical mysteries.
Ready to accelerate your research?
Start your journey with Lifebit’s rare disease AI and join the mission to ensure no rare disease remains undiagnosed or untreated.

