How AI is changing the way clinical providers make decisions

AI Clinical Decision: Cut Errors by 30% and Save 3 Hours Weekly
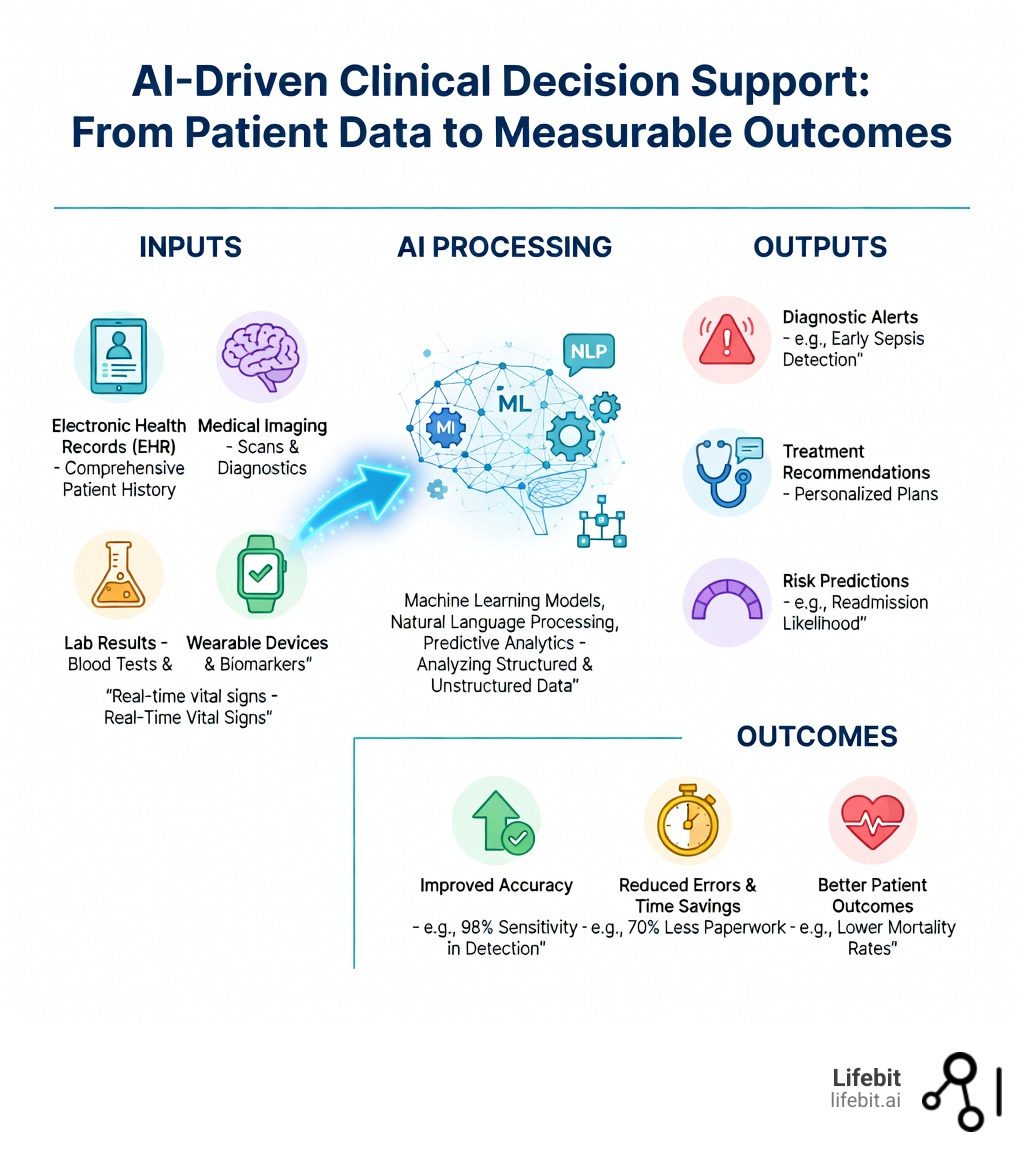
AI clinical decision support systems are using machine learning, natural language processing, and predictive analytics to help physicians diagnose diseases, predict patient outcomes, and personalize treatments—often reducing diagnostic errors by 30% or more while cutting administrative time by over 70%.
What AI Clinical Decision Support Does:
- Enhances diagnostic accuracy by analyzing imaging, lab results, and EHR data (e.g., 97.2% sensitivity for detecting venous thromboembolism, 98.6% accuracy for heart valve disease)
- Reduces cognitive load by automating documentation and flagging drug interactions in real time
- Predicts patient deterioration such as sepsis onset, hospital readmissions, and acute kidney injury before symptoms escalate
- Personalizes treatment plans by integrating genomics, clinical notes, and real-time monitoring data
- Saves clinicians over 3 hours per week on paperwork through AI scribes and automated clinical note generation
Healthcare systems worldwide face unprecedented challenges: exploding data volumes, rising complexity, and resource constraints. Traditional clinical decision-making—relying on human memory, experience, and fragmented data sources—can miss subtle patterns, introduce cognitive bias, and lead to preventable errors.
AI changes that.
By processing structured and unstructured data from electronic health records, medical imaging, lab results, and even real-time wearable devices, AI systems provide evidence-based recommendations at the point of care. These tools don’t replace clinicians—they augment human expertise, offering a “second opinion” backed by millions of data points and peer-reviewed evidence.
The impact is already measurable. Studies show AI-driven clinical decision support can achieve diagnostic accuracy comparable to trained specialists in fields like dermatology, radiology, and pathology. Early warning systems for sepsis have reduced mortality rates. AI scribes have cut documentation time by 70%, allowing physicians to focus on patients instead of paperwork.
But the promise of AI in clinical decision-making goes beyond efficiency. It’s about equity, safety, and scale—enabling smaller clinics to access the same level of diagnostic support as major academic centers, reducing health disparities, and ensuring that every patient benefits from the latest medical evidence.
As the CEO and Co-founder of Lifebit, I’ve spent over 15 years working at the intersection of computational biology, AI, and health-tech entrepreneurship, building platforms that enable secure, federated analysis of biomedical and genomic data. Throughout this work, I’ve seen how AI clinical decision tools can transform patient care when implemented responsibly, with robust data governance, clinical validation, and physician trust at the center. This guide will walk you through the core capabilities, real-world impact, implementation challenges, ethical guardrails, and future opportunities of AI in clinical decision-making—so you can understand how this technology is reshaping healthcare and what it means for your organization.
Key AI clinical decision vocabulary:
AI Clinical Decision: Fix Diagnostic Blind Spots with Multimodal Data
In the context of modern medicine, Artificial Intelligence (AI) isn’t a single “robot doctor” but a suite of core capabilities designed to process information faster and more accurately than the human brain. At its heart, AI clinical decision support involves using machine learning (ML) and natural language processing (NLP) to turn massive, messy datasets into actionable insights. This transformation is critical because the volume of medical data is doubling every 73 days, making it impossible for even the most dedicated clinicians to stay current without technological assistance.

The Three Pillars of AI-Driven Support
To understand how AI functions at the point of care, we must look at its three primary functional pillars:
- Diagnostic Support and Computer Vision: AI-powered systems utilize Convolutional Neural Networks (CNNs) to analyze medical images (MRI, CT, X-ray) with superhuman consistency. These tools identify abnormalities, such as malignant nodules, micro-fractures, or early-stage hemorrhages, often with higher precision than human radiologists who may be suffering from fatigue. For instance, AI can scan thousands of pathology slides to find a single cluster of cancer cells that might be overlooked during a manual review.
- Prognostic Tools and Predictive Analytics: By evaluating historical patient data alongside real-time vitals, AI can predict the likelihood of future adverse events. This includes calculating the risk of hospital readmission within 30 days or identifying patients at high risk for cardiovascular events. These models look at non-obvious correlations, such as how a slight change in sleep patterns combined with a specific lab value might signal an impending crisis.
- Therapeutic Decisions and Precision Medicine: AI helps clinicians move away from the “one-size-fits-all” approach. By integrating genomic data with clinical history, AI can suggest which chemotherapy regimen is most likely to be effective for a specific genetic mutation, or which dosage of an anticoagulant is safest for a patient based on their metabolic profile.
The Power of Multimodal Data Integration
What makes modern AI truly revolutionary is its ability to handle multimodal data. In the past, clinical decision support was siloed—one tool for labs, another for imaging. Modern systems can synthesize structured data (blood pressure, glucose levels, heart rate) with unstructured data (clinical notes, genomic sequences, and medical imaging). This creates a holistic, 360-degree view of the patient. For example, an AI might flag a patient for a rare autoimmune disorder by connecting a specific phrase in a physician’s note from three years ago with a recent abnormal lab result and a subtle pattern on a new chest X-ray. This level of synthesis was previously impossible to achieve manually. For a deeper dive into how these technologies work, see our guide on AI-Powered Diagnostics.
AI Clinical Decision: Why Rule-Based Systems Fail and What’s Next
The journey of AI in healthcare didn’t start with deep learning; it began with “if-then” logic. Understanding this evolution helps us appreciate the power of today’s systems and why the shift to machine learning was necessary for patient safety.
The Era of Rule-Based Expert Systems
Early Clinical Decision Support Systems (CDSS), developed in the 1970s and 80s (like the famous MYCIN system), were powered by rule engines. These systems relied on a rigid set of predefined rules created by medical experts. For example: If a patient’s blood sugar is over 200 mg/dL and they haven’t eaten, then flag for potential diabetes.
While these were a step forward, they suffered from several fatal flaws:
- Brittleness: If a patient case didn’t fit the exact parameters of the rule, the system failed.
- Maintenance Burden: As medical knowledge evolved, thousands of rules had to be manually updated by hand, which was unsustainable.
- Alert Fatigue: Because they lacked nuance, these systems often triggered hundreds of low-priority alerts, leading doctors to ignore them entirely.
According to Scientific research on rule engines in medicine, while 46.4% of research in this area still originates from the U.S., these systems are increasingly being replaced or integrated into “hybrid” models that combine expert knowledge with machine learning flexibility.
The Shift to Machine Learning and Deep Learning
Today, we’ve moved from SQL-based data management to predictive analytics. Modern machine learning doesn’t need to be told the rules; it discovers them by analyzing millions of patient outcomes. This is the difference between a system that knows what a cat looks like because you told it “cats have ears” and a system that knows what a cat looks like because it has seen a billion photos of cats.
| Feature | Rule-Based Systems (Old) | Machine Learning (New) |
|---|---|---|
| Logic | Predefined “If-Then” rules | Learned from data patterns |
| Data Handling | Primarily structured data (SQL) | Multimodal (Images, Text, Genomics) |
| Adaptability | Hard to update; static | Continuously learns from new outcomes |
| Complexity | Best for simple, clear-cut logic | Best for complex, “fuzzy” diagnostics |
| Scalability | Limited by human input | Limited only by compute and data |
Recent temporal trends show a massive spike in deep learning applications post-2020, particularly in the use of Transformers and Large Language Models (LLMs) to parse clinical documentation. We are seeing a global distribution of this technology, with high-income countries leading the way, though the goal is to make these tools accessible everywhere through federated networks that allow models to be trained on diverse global populations without moving sensitive data.
AI Clinical Decision: 98% Accuracy and 70% Less Paperwork
The most exciting part of AI clinical decision tools is seeing them work in real hospital settings. The statistics are no longer theoretical; they are measurable milestones in patient care and operational efficiency.
Sepsis and Critical Care: The Golden Hour
Sepsis is a leading cause of hospital death, largely because every hour of delay in treatment increases the risk of mortality by nearly 8%. AI CDSS tools are now bringing life-saving possibilities to the ICU by acting as an early warning system. One landmark study showed that AI-driven algorithms could predict sepsis onset up to 12 hours before clinical symptoms appeared. By analyzing subtle changes in heart rate variability, respiratory rate, and white blood cell counts, the AI alerts the nursing staff to begin antibiotic protocols during the “golden hour,” significantly reducing mortality rates and length of stay.
Administrative Relief and the “Scribe” Revolution
One of the biggest pain points for doctors is the “paperwork tax.” For every hour spent with a patient, physicians often spend two hours on electronic health record (EHR) documentation. This is a primary driver of burnout. Scientific research on AI scribes reducing administrative load found that AI scribes—which use ambient listening to draft clinical notes in real-time—led to a 70% reduction in time spent on paperwork. That is over 3 hours per week saved on administrative tasks, which can now be spent face-to-face with patients, improving the patient-provider relationship.
Diagnostic Accuracy Milestones Across Specialties
AI isn’t just faster; it’s often more accurate in specific, high-stakes domains:
- VTE Detection: Rule engines and NLP used on pediatric radiology reports achieved a 97.2% sensitivity for identifying new venous thromboembolism, ensuring that at-risk children receive immediate intervention.
- Heart Valve Disease: Integrated EHR platforms using AI identified patients with heart valve disease with 98.6% accuracy, catching cases that were missed during standard physical exams.
- Oncology and Breast Cancer: AI-powered tools showed 66% congruency with physician decisions but helped identify subtle metastases in lymph nodes that humans missed. In some trials, AI reduced the false positive rate in mammography by 5.7%, sparing thousands of women from unnecessary biopsies.
- Diabetes Management: Approximately 30% of recent research focuses on improving A1C levels through automated insulin adjustment. AI models can predict glucose fluctuations based on diet, exercise, and sleep, providing real-time dosing recommendations that prevent dangerous hypoglycemic events.
AI Clinical Decision: Stop the ‘Black Box’ Problem and Build Trust
Despite these wins, the road to full adoption has significant speed bumps. The biggest hurdle isn’t the technology itself, but clinician trust and the “socio-technical” challenge of integrating AI into a high-pressure workflow.
The Challenge of Explainability (XAI)
Many advanced AI models, particularly deep neural networks, are “black boxes”—they give a recommendation without explaining why. For a doctor responsible for a patient’s life, “the computer said so” isn’t an acceptable answer. If an AI flags a patient for a rare lung disease, the doctor needs to see which features in the CT scan or which lab values triggered that alert. This is why Scientific research on responsible machine learning for healthcare emphasizes the need for explainable AI (XAI). XAI techniques, such as saliency maps or attention mechanisms, highlight the specific data points the AI used to reach its conclusion, allowing the clinician to verify the logic.
Interoperability and the Data Silo Problem
Other major challenges include:
- Interoperability: Different hospitals use different EHR vendors (Epic, Cerner, etc.) and different data formats. If the AI can’t “talk” to the EHR or pull data from a third-party lab, it’s useless. The industry is moving toward FHIR (Fast Healthcare Interoperability Resources) standards, but the transition is slow.
- System Complexity and Workflow Integration: Adding another tool to a busy clinician’s workflow can increase cognitive load rather than reduce it. If a doctor has to log into a separate portal to see AI insights, they won’t use it. The AI must be embedded directly into the existing EHR interface.
- Data Quality and Bias: If the input data is biased (e.g., if a model is trained only on patients from one geographic region), the AI clinical decision will be flawed when applied to a diverse population.
At Lifebit, we address these issues through our AI-Enabled Data Governance Ultimate Guide, ensuring that data is harmonized, cleaned, and secure before the AI even touches it.
Evaluating the Safety of an AI Clinical Decision System
How do we know a system is safe? Healthcare organizations must use a rigorous validation framework before deployment. This involves “shadow testing,” where the AI runs in the background without influencing care, allowing administrators to compare its recommendations against actual physician decisions over several months.
Key Evaluation Metrics for AI-CDSS:
- Sensitivity and Specificity: How often does it catch the disease (sensitivity) vs. how often does it give a false alarm (specificity)?
- Algorithmic Transparency: Can the system “show its work” through XAI?
- Clinical Validation: Has it been tested in a real-world environment with diverse patient demographics, not just on a clean, curated dataset?
- Model Drift Detection: Does the AI’s accuracy decrease over time as medical practices change or as the patient population shifts? Continuous monitoring is required to ensure the model remains calibrated.
AI Clinical Decision: Protect Privacy Without Losing Speed
As we integrate AI clinical decision tools, we must answer tough questions about accountability and ethics. The transition from human-led to AI-augmented care creates a new landscape of legal and moral responsibility.
The Liability Gap: Who is Responsible?
If an AI makes a mistake—or if a doctor follows an AI’s incorrect recommendation—who is liable? The doctor? The software developer? The hospital? Current Scientific research on ethics and reliability in AI points to several critical guardrails. Most legal frameworks currently treat AI as a “learned intermediary” tool, similar to a medical textbook or a calculator. This means the physician remains the ultimate decision-maker. However, as AI becomes more autonomous, this legal definition is being challenged, leading to calls for new insurance models specifically for AI-driven healthcare.
Data Privacy and the Federated Solution
Patient data is the most sensitive information an individual owns. It must be protected under strict regulations like HIPAA in the U.S. and GDPR in Europe. Traditionally, training an AI required moving all patient data to a central server, which created massive security risks.
Federated Learning is the solution. In a federated model, the AI travels to the data. The model is trained locally at the hospital, and only the “learned insights” (mathematical weights) are sent back to the central system. The actual patient records never leave the hospital’s secure firewall. This allows for global collaboration on rare diseases without ever compromising individual privacy.
Addressing Algorithmic Bias
If an AI is trained only on data from one demographic, it may perform poorly for others. For example, a skin cancer detection AI trained primarily on light-skinned patients may have lower accuracy for patients with darker skin tones. To ensure equity, we must:
- Audit models for fairness: Regularly testing performance across different age, gender, and ethnic groups.
- Diverse Data Sourcing: Ensuring training sets are representative of the global population.
- Patient Consent: Patients have a right to know if an AI is involved in their diagnosis and how their data is being used to improve the system.
Ultimately, AI is a support tool, not a replacement. The goal is “Human-in-the-loop” AI, where the machine handles the data crunching and the human provides the empathy, ethical judgment, and final clinical sign-off.
AI Clinical Decision: Real-Time Monitoring Beyond the Hospital
The next frontier for AI clinical decision support is moving from the hospital to the home, creating a continuous loop of care that prevents crises before they happen.
The Rise of the Internet of Medical Things (IoMT)
We are entering the era of real-time monitoring. Imagine a single system instance handling 1,000 personal medical devices (PMDs) simultaneously. These devices—smartwatches, continuous glucose monitors, and smart inhalers—generate a constant stream of data. If a patient’s heart rate variability or respiratory characteristics show early signs of heart failure or an asthma attack, the AI flags it to the clinician immediately. This allows for “proactive intervention”—potentially days before the patient would have felt sick enough to go to the ER.
This is the heart of AI in Personalized Medicine. By combining real-world evidence from wearables with multi-omic data (genomics, proteomics, metabolomics), we can predict hospital readmission risk with unprecedented accuracy and tailor drug dosages to a patient’s specific genetic profile in real-time.
Digital Twins and Predictive Simulation
One of the most futuristic applications of AI in clinical decision-making is the concept of the Digital Twin. A digital twin is a virtual representation of a patient’s physiology. Clinicians can use AI to “test” a treatment on the digital twin before giving it to the actual patient. For example, a surgeon could simulate a complex heart surgery on a 3D digital model to predict how the patient’s specific anatomy will react, or an oncologist could simulate how a tumor might shrink in response to different combinations of immunotherapy.
Future Opportunities for Global Public Health
The opportunities are expanding into every corner of public health:
- Genomics at Scale: Integrating DNA data to predict lifetime disease risk through AI for Genomics.
- Population Health Management: Identifying at-risk communities before a health crisis spreads by analyzing social determinants of health alongside clinical data.
- Global Federated Networks: Allowing a small clinic in a developing nation to benefit from the collective “intelligence” of the world’s leading academic medical centers without needing to build their own massive data infrastructure.
- Real-Time Evidence (RTE): Using “live” data from millions of patients to update clinical guidelines in weeks rather than the years it takes for traditional clinical trials to be published.
AI Clinical Decision FAQ: Solving Privacy, Bias, and Burnout
How does AI improve diagnostic accuracy in clinical settings?
AI analyzes patterns across millions of data points—including images, lab results, and clinical notes—that are often too subtle for the human eye. For example, deep learning models can detect skin cancer or diabetic retinopathy with accuracy levels that match or exceed trained specialists.
What are the primary ethical concerns regarding AI in healthcare?
The big three are privacy, bias, and accountability. There is a risk that AI could leak sensitive data, perpetuate racial or gender biases found in historical data, or create “liability gaps” where it’s unclear who is responsible for a machine-generated error.
Can AI clinical decision tools reduce physician burnout?
Yes! By automating documentation through AI scribes (saving ~3 hours/week) and streamlining the search for clinical evidence, AI reduces the administrative burden and cognitive load, allowing doctors to focus on the human side of medicine.
AI Clinical Decision: Start Saving Lives and Time Today
The integration of AI clinical decision support is no longer a futuristic concept—it is a present-day reality that is saving lives and restoring time to the medical profession. From detecting sepsis in the ICU to reducing paperwork by 70%, AI is the “second opinion” that never gets tired and never stops learning.
However, the true power of AI is only unlocked when data is accessible, secure, and harmonized. At Lifebit, our federated AI platform enables this by providing secure, real-time access to global biomedical and multi-omic data. We help biopharma, governments, and public health agencies turn massive datasets into life-saving clinical insights without ever compromising patient privacy.
Ready to see how federated AI can power your clinical decisions? Explore the Lifebit Platform.

