AlphaGenome and Beyond: Your Guide to Top AI in Genomics

Why the Best AI for Genomics Matters Now
The best AI for genomics includes tools that can reduce variant calling errors by over 50%, achieve up to 98% accuracy in prioritizing clinically relevant variants, and analyze up to 1 million DNA letters at once. These advanced AI systems are changing how researchers decode the genome—from identifying disease-causing mutations to designing novel therapeutic candidates.
Top AI capabilities for genomics at a glance:
- Large-Scale Sequence Processing: Process up to 1 million DNA letters with single-nucleotide resolution, outperforming previous models in multiple evaluations.
- Generative DNA Models: Generate novel DNA sequences over 1 million base pairs long, trained on millions of genomes.
- High-Accuracy Variant Prediction: Predict variant pathogenicity with up to 95% accuracy, leveraging data from hundreds of species.
- Non-Coding Variant Analysis: Identify non-coding promoter variants, uncovering previously hidden rare disease drivers.
- RNA Foundation Models: Predict sub-gene resolution effects to accelerate the development of RNA therapeutics.
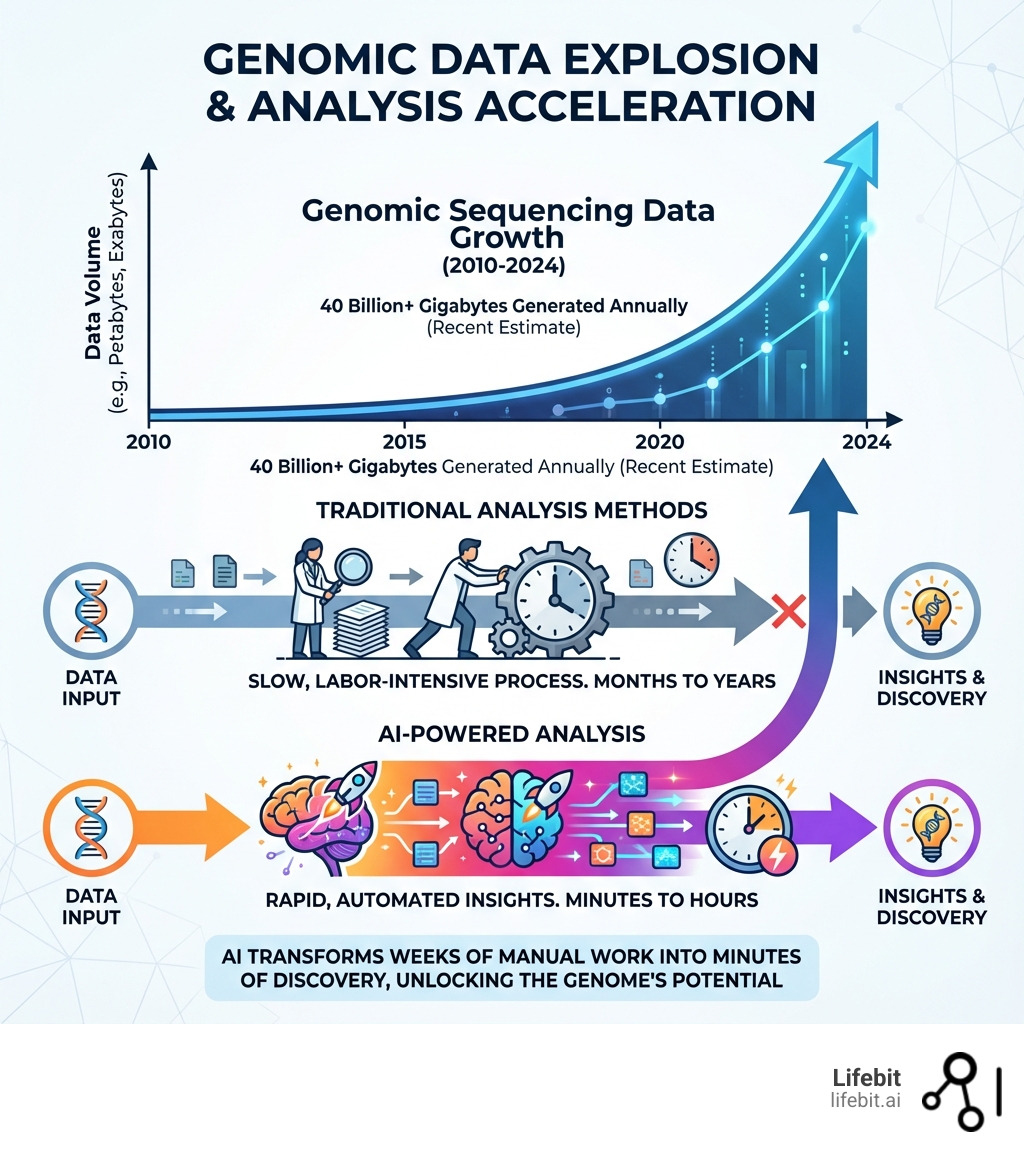
The genomic data explosion is real. The industry now generates upward of 40 billion gigabytes of genomic data every year. Yet the function of 99.9% of human genetic variants remains unknown. With over 98% of the genome being non-coding, manual analysis simply can’t keep pace.
AI is changing that. Modern genomic AI can deliver insights in minutes that once took human analysts weeks or months. It’s not just about speed—AI is uncovering patterns humans would never find. In one study of over 2,300 cancer patients, AI found disease-causing variants in 14% more individuals than previous methods.
This guide will walk you through the leading AI platforms and tools reshaping genomics research. You’ll learn how foundation models are learning the “language” of DNA, which tools excel at variant calling versus drug findy, and how to prepare your data for AI-ready analysis. We’ll also explore the ethical considerations and future breakthroughs on the horizon.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, a federated genomics and biomedical data platform powering AI-driven findy across secure, compliant environments. With over 15 years in computational biology and AI, I’ve seen how the best AI for genomics can transform research when paired with federated data access and robust infrastructure.
Best ai for genomics terms made easy:
Why Genomics Needs AI: Taming the Data Tsunami
Imagine trying to read every book in the Library of Congress, but most of the pages are blank, and the few with text are in a language you only partially understand. That’s a bit like genomics without AI. The sheer volume and complexity of genomic data generated today are staggering, reaching petabyte scales globally. While it once took 13 years and billions of dollars to map the first human reference genome, we can now sequence genomes in hours for a fraction of the cost. The problem isn’t getting the data anymore; it’s making sense of it all.
AI steps in as our super-powered librarian, capable of instantly scanning, indexing, and understanding this massive biological library. It transforms what would be months or years of manual analysis into minutes. This not only dramatically speeds up research and clinical breakthroughs but also reduces human error, allowing us to dig into the vast, often overlooked, non-coding regions that make up 98% of our genome. These “dark genome” areas, once mysteries, are now yielding crucial insights into gene activity and disease.

The Core Challenges Lifebit’s AI Solves
At Lifebit, we’re dedicated to tackling the fundamental problems in genomic research with our advanced AI. We aim to transform how researchers interpret the genome, from identifying disease-causing mutations to designing novel therapeutic candidates. Here’s how our AI helps address the core challenges:
- Reducing Variant Calling Errors: Identifying genetic variants is like assembling a massive, error-prone jigsaw puzzle. Traditional methods often miss subtle but critical pieces. Our AI-powered tools significantly reduce variant calling error rates by over 50%, ensuring more accurate detection of genetic variations.
- Accelerating Drug Findy: Developing new drugs is a decade-long, multi-billion-dollar process with a high failure rate. Our AI accelerates this by identifying promising therapeutic targets, designing novel drug candidates, predicting their behavior in different tissues, and even creating surrogate molecules for testing. This drastically cuts down on the time and cost involved in bringing life-saving treatments to patients.
- Interpreting Non-Coding DNA: The non-coding regions, making up 98% of our genome, are crucial for orchestrating gene activity, yet their function is largely unknown. Our AI acts as a guide, finding new biological functions in these vast, unmapped regions and linking non-coding variants to disease mechanisms. For instance, our tools can predict the impact of non-coding promoter variants that disrupt gene expression, uncovering previously hidden genetic drivers of rare diseases.
- Integrating Multi-Omics Data: Biological systems are complex, involving multiple layers of data—genomics, transcriptomics, epigenetics, and proteomics. Our AI excels at integrating and analyzing these diverse ‘omics’ layers, providing a holistic view of biological processes that single-omic analyses simply can’t achieve.
- Breaking Down Barriers to Personalized Medicine: Each of us carries about 4 million genetic variants, 99.9% of which have unknown functions. Our AI analyzes an individual’s genetic blueprint to predict disease risks years in advance, shifting healthcare from reactive to proactive. By accurately matching treatments to individual genetic profiles, we’re paving the way for truly personalized and precision medicine.
The Lifebit Advantage: AI Models Powering Genomic Findy
At Lifebit, we understand that the power of AI in genomics is not just about raw computational strength; it’s about secure, compliant, and insightful analysis of sensitive data. Our federated AI platform is designed to provide next-generation capabilities, enabling secure, real-time access to global biomedical and multi-omic data. With built-in features for data harmonization, advanced AI/ML analytics, and federated governance, we empower large-scale, compliant research and pharmacovigilance across biopharma, governments, and public health agencies across the USA, Canada, Europe, the UK, Israel, Singapore, and beyond. Our platform, including components like the Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), delivers real-time insights and secure collaboration across hybrid data ecosystems.
Our approach centers on how our AI models learn the “language” of DNA. Just as large language models (LLMs) process text, our genomic foundation models are learning to “read” DNA sequences like sentences, understanding context and relationships in unprecedented ways. This allows us to develop advanced models for variant effect prediction, gene expression, and much more.
Lifebit’s AI for Decoding Gene Regulation
Understanding gene regulation is paramount to deciphering disease. Our AI models are at the forefront of this effort, providing unparalleled accuracy and scale:
- Predicting Variant Impacts with High Accuracy: Our AI platforms leverage advanced models that can process up to 1 million DNA letters, making predictions at the resolution of individual letters. This allows us to accurately predict the regulatory effect of a variant, matching or exceeding the top-performing external models on 24 out of 26 evaluations. Modern AI systems achieve up to 98% accuracy in prioritizing clinically relevant variants.
- Analyzing Millions of DNA Letters at Scale: Genomic foundation models within our ecosystem are capable of processing vast stretches of DNA—up to 1 million base pairs at once—to understand their regulatory activity. This long sequence-context at high resolution is crucial for capturing the intricate interplay of genetic elements.
- Understanding Regulatory Processes: The non-coding regions of the genome, though not coding for proteins, are vital for orchestrating gene activity. Our AI explicitly models RNA splicing junctions directly from sequence, offering deeper insights into genetic diseases caused by splicing errors.
- Outperforming Traditional Approaches: By integrating sophisticated AI, we consistently see our tools outperform traditional methods in accuracy and speed across various genomic tasks, leading to faster, more reliable findings.
Generative AI for Writing and Optimizing Genetic Code
The ability to not just read but also “write” genetic code represents a paradigm shift in biology. Our platforms facilitate this through generative AI, opening new frontiers in synthetic biology and gene editing.
- AI-Driven Design of Novel DNA Sequences: Generative AI models can be leveraged within our ecosystem to write genetic code. For instance, some models can generate DNA sequences of more than 1 million base pairs, surpassing the size of many bacterial genomes. This capability allows researchers to design entirely new biological systems or optimize existing ones.
- Applications in Synthetic Biology and Gene Editing: This generative power has profound implications. Researchers can use AI to understand microbial and viral genomes, fashion new proteins (drugs), and even reprogram microbes for tasks like carbon sequestration or microplastic cleanup. AI can also codesign protein-RNA systems, such as novel CRISPR-Cas systems, accelerating the development of precise gene-editing tools.
- Large Context Windows for Complex Sequence Analysis: These generative models benefit from vast context windows, processing sequences up to 131,000 base pairs, or even up to 1 million DNA letters, to understand and generate complex genetic instructions.
- Accelerating the Design-Build-Test Cycle: By rapidly generating and evaluating potential genetic constructs, AI significantly accelerates the traditional design-build-test cycle in synthetic biology, allowing researchers to focus on promising possibilities rather than relying on brute-force testing or unpredictable mining from nature.
Other Powerful Tools in the Lifebit Ecosystem
Our federated AI platform is designed to be a comprehensive ecosystem, enabling researchers to integrate and leverage a wide array of advanced AI tools. This includes:
- Advanced Variant Calling Algorithms: Leveraging machine learning to detect single nucleotide variants (SNVs), insertions/deletions (indels), and structural variants with improved sensitivity and precision.
- Gene Expression Prediction Models: AI that can predict RNA expression patterns with incredible detail, offering insights into gene activity at sub-gene resolution and opening new avenues for RNA-based therapeutics.
- Multi-Omics Integration Tools: Sophisticated AI for analyzing and integrating data across genomics, transcriptomics, epigenetics, and proteomics, providing a holistic understanding of biological systems.
- Drug Target Identification and Design Tools: AI-powered platforms that can identify promising therapeutic targets, design new drug candidates, and predict their behavior, accelerating the drug findy pipeline.
- Secure Collaboration and Analytics: All these tools are deployed within a secure, compliant framework that enables researchers to collaborate globally on sensitive datasets without compromising data privacy or security.
From Code to Cure: Real-World Impact of Lifebit’s AI in Genomics
The integration of AI into genomics is not just theoretical; it’s delivering tangible, measurable improvements in patient outcomes and accelerating biological findy. With Lifebit’s platform, we’re changing raw genomic sequences into actionable clinical insights.

Supercharging Precision Medicine and Variant Calling
In the field of precision medicine, AI is a game-changer:
- Boosting Variant Prioritization Accuracy: Our AI systems achieve up to 98% accuracy in prioritizing clinically relevant variants, ensuring that researchers and clinicians can quickly focus on the most impactful genetic changes.
- Identifying More Disease-Causing Variants: In a study of over 2,300 cancer patients, AI found disease-causing variants in 14% more individuals than previous methods, leading to more targeted treatments. AI can also reduce the time to diagnose rare diseases from months to days.
- Cutting Variant Calling Errors: AI-powered tools consistently reduce variant calling error rates by over 50%, enhancing the reliability of genomic diagnostics.
- Clinical Decision Support and Patient Stratification: By analyzing an individual’s genetic blueprint, our AI can predict disease risks years in advance, shifting healthcare from reactive to proactive. It enables precise patient stratification, matching treatments to individual genetic profiles through secure, federated analysis, and supports clinical decision-making with high confidence.
Decoding the “Dark Genome”: Lifebit’s AI in Non-Coding Regions
The vast non-coding regions of our genome, often called the “dark genome,” hold immense potential for understanding disease. Our AI is illuminating these previously inscrutable areas:
- Gene Expression Prediction: Our AI models excel at predicting thousands of molecular properties that characterize regulatory activity and RNA expression at sub-gene resolution. This deep understanding of gene regulation is critical for identifying novel therapeutic interventions.
- Identifying Improvers and Promoters: AI can pinpoint non-coding promoter variants that disrupt gene expression, uncovering up to 6% of previously hidden genetic drivers of rare diseases. These insights are vital for understanding how genetic variations influence health and disease.
- Understanding Long-Range Interactions: With the ability to analyze up to 1 million base pairs of DNA sequence at high resolution, our AI captures long-range interactions between regulatory elements and genes, which are often missed by traditional methods.
- Linking Non-Coding Variants to Disease: By connecting specific non-coding variants to disease genes, our AI helps investigate the potential mechanisms of complex conditions, such as cancer-associated mutations, offering a more complete picture of disease etiology.
Engineering Life with Synthetic Biology and Gene Editing
The ability to design and engineer biological systems is being revolutionized by AI:
- Designing Functional DNA: Generative AI models within our ecosystem can “write” genetic code, enabling the design of novel, functional DNA sequences for various applications, from optimized protein production to creating new cellular pathways.
- Optimizing CRISPR Guide RNAs: AI plays a crucial role in enhancing the precision of gene editing. It can codesign novel CRISPR-Cas systems and optimize guide RNAs, making genetic modifications with near-perfect accuracy and reducing off-target effects.
- Accelerating the Design-Build-Test Cycle: By rapidly generating and evaluating potential genetic constructs in silico, AI dramatically speeds up the iterative design-build-test cycle in synthetic biology, changing years of experimental trial-and-error into months or even weeks.
- AI-Driven Innovation in Gene Editing: This AI-driven approach is fostering innovation in gene editing, enabling the development of more effective and safer therapeutic strategies for genetic diseases.
Your AI-Ready Checklist: Preparing Genomic Data for Analysis
Before we release the power of AI, we must first ensure our data is ready. The old adage “garbage in, garbage out” has never been more true than in AI-driven genomics. Even the best AI for genomics is only as good as the data it’s trained on. High-quality, well-prepared data is non-negotiable for ensuring reproducibility, accuracy, and ultimately, powerful AI insights.
Real-world genomics data can be messy, riddled with errors, duplicates, and inconsistencies. Unprepared data can severely hamper scientific progress and lead to misleading AI predictions. By carefully preparing our data, we set the stage for AI to uncover truly meaningful biological findies.
5 Steps to Prepare Genomic Data for AI
To open up the full potential of AI in your genomic research, we recommend these five essential steps for data preparation:
- Clean Up Your Data: This is the first and most critical step. We must correct errors, remove duplicates, and fix missing values. Thorough data cleaning involves identifying and addressing anomalies or inconsistencies that could otherwise derail AI model training and lead to flawed conclusions.
- Standardize and Harmonize: Genomic data comes in various formats. We need to convert raw sequence reads and other unstructured data into standardized formats like FASTA files for biological sequences or BAM files for DNA sequence alignments. Crucially, we must address batch effects—technical variations that can creep in from different sample processing conditions—using advanced correction techniques.
- Structure and Annotate: Organize your data into a machine-readable format and link genomic features, such as genes and regulatory elements, to relevant biological traits and health outcomes. This often involves clear annotation and labeling, combining computational tools with expert manual curation for the highest accuracy.
- Ensure Diversity and Balance: AI models perform best on large, varied datasets. We must train models on diverse samples to avoid “overfitting,” where an AI model trains too specifically to a target dataset and performs poorly on new, unseen data. Addressing imbalances—for instance, between healthy and diseased samples or across different ancestral populations—is vital to prevent biased results. This can involve adding external data, generating synthetic data, or using resampling techniques.
- Track Provenance and Ensure Accessibility: Maintain a clear record of your data’s origin and every processing step. This “data provenance” ensures reproducibility and provides crucial information on data quality. Adherence to the FAIR Guiding Principles for data management (Findable, Accessible, Interoperable, Reusable) is paramount for ensuring that your data can be easily used by both machines and human researchers, fostering collaboration and scientific rigor.
Scaling Up: How Lifebit Handles Genomics at Scale
At Lifebit, we understand that preparing data is only half the battle; analyzing it at scale is the other. Our platform is built from the ground up for massive genomic datasets, leveraging high-performance computing (HPC) and cloud-native solutions.
We enable secure, federated analysis across borders, allowing researchers in London, New York, across Europe, and Singapore to collaborate on petabyte-scale datasets without physically moving the sensitive data. This is achieved through our federated AI platform, which brings the analysis to the data, ensuring privacy and compliance with stringent regulations. Our workflow automation and distributed computing capabilities further streamline pre-processing and analysis, making large-scale genomic research not only possible but efficient.
The Road Ahead: Future Breakthroughs and Ethical Guardrails
As we push the boundaries of AI in genomics, we recognize the critical importance of balancing innovation with responsibility. The future of biological findy promises breakthroughs that will reshape medicine and our understanding of life itself, but we must steer this path with careful ethical consideration.
Navigating the Ethical Maze
Genomic data is profoundly sensitive, and its use in AI raises important ethical questions:
- Data Privacy and Security: The highly personal nature of genomic information necessitates robust data privacy and security measures. Our federated learning approach is a game-changing solution, allowing AI models to train across distributed, sensitive datasets without directly accessing the raw data, ensuring privacy and compliance.
- Eliminating Algorithmic Bias: Historically, many genomic datasets have disproportionately focused on populations of European ancestry. This can lead to AI models that perform poorly or are biased against underrepresented populations. We are committed to ensuring representative datasets and developing algorithms that are less affected by ancestry bias, promoting equitable precision medicine for all.
- Ensuring Representative Datasets: Actively working to include diverse datasets and apply techniques to balance them is crucial for building AI models that are fair and generalize across all populations.
- Federated Learning as a Solution: Our federated governance framework, powering large-scale, compliant research, offers a robust mechanism for secure collaboration across hybrid data ecosystems, adhering to principles like the GA4GH standards for responsible data sharing.
Future Trends and Potential Breakthroughs
The synergy between AI and genomics is just beginning to unfold, promising exciting developments:
- Multi-Modal Models: Expect to see increasingly sophisticated AI models that integrate not just genomic data, but also proteomics, transcriptomics, and epigenomics. These multi-modal AI systems will provide a more comprehensive understanding of biological systems.
- AI-Driven Experimental Design: AI will move beyond analysis to actively design experiments, optimizing parameters and predicting outcomes, accelerating the pace of findy in wet labs. Imagine AI automating wet-lab experimental design, reducing trial-and-error significantly.
- Generative Biology for Novel Therapeutics: Generative AI will continue to advance, enabling the de novo design of novel proteins, RNA sequences, and even entire genetic pathways for therapeutic purposes, leading to breakthroughs in drug development.
- In-Silico Clinical Trials: The ability of AI to simulate biological processes and predict drug responses will pave the way for virtual clinical trials, drastically reducing the time, cost, and ethical complexities of traditional trials.
- Autonomous Labs: We envision a future where AI-powered robots and systems can conduct experiments, analyze data, and even formulate new hypotheses with minimal human intervention, creating fully autonomous research pipelines.
Frequently Asked Questions about AI in Genomics
What is a genomic foundation model?
A genomic foundation model is a very large-scale artificial intelligence model that has been pre-trained on immense quantities of DNA sequence data. Its purpose is to learn the fundamental patterns, syntax, and relationships within biological code. Much like large language models learn human language, these models understand the “language” of DNA. Once pre-trained, these powerful models can then be fine-tuned for a wide range of specific tasks, such as predicting the impact of genetic variants, analyzing gene expression levels, or even generating novel DNA sequences for synthetic biology applications. They represent a significant leap forward in our ability to interpret and engineer life.
Can AI replace human geneticists?
No, AI is a powerful tool designed to augment, not replace, human expertise. While AI excels at processing massive datasets, identifying subtle patterns, and automating repetitive tasks, human geneticists remain crucial. They provide the clinical context, interpret complex AI outputs, design experiments, and, critically, ensure ethical oversight. The best AI for genomics acts as a brilliant co-pilot, handling the heavy computational lifting and surfacing insights, while the human geneticist remains the captain, making informed decisions and guiding the research or clinical application.
How can I start using AI for my genomics research?
Starting your journey with AI in genomics begins with a solid foundation: ensure your genomic data is clean, well-structured, and adheres to FAIR principles. For seamless and secure integration of AI into your research, we recommend exploring Lifebit’s secure, scalable platform. Our platform is specifically designed to handle large-scale genomic data, providing the necessary high-performance computing (HPC) and cloud-native solutions for robust AI training and analysis. We encourage collaboration with data scientists and bioinformaticians, as their expertise is invaluable in leveraging these powerful tools effectively.
Conclusion
The genomic data explosion has made one thing abundantly clear: AI is no longer a futuristic concept in genomics; it is an essential, present-day necessity. The sheer volume and complexity of DNA, RNA, and multi-omic data demand intelligent solutions to extract meaningful insights. We’ve seen how the best AI for genomics can dramatically accelerate findy, reduce errors, and open up the secrets of the non-coding genome, from variant calling to drug findy and precision medicine.
At Lifebit, our secure, federated AI platform is at the forefront of this revolution, enabling compliant, real-time access to global biomedical data and powering advanced AI/ML analytics. We believe the key to success lies in high-quality, AI-ready data combined with secure, scalable analysis capabilities. Lifebit empowers you to overcome data silos, collaborate globally, and confidently open up the full potential of your genomic data, driving the next era of biological findy and improving human health worldwide.

