9 Best Trusted Research Environment Vendors for Secure Health Data Analysis in 2026

Government health agencies and biopharma R&D teams face the same problem: sensitive genomic and clinical data locked in silos, wrapped in compliance requirements, and impossible to analyze at scale. Trusted Research Environments (TREs) solve this by providing secure, compliant workspaces where researchers access data without moving it.
But not all TRE vendors are equal. Some excel at federated analysis across borders. Others specialize in rapid deployment or AI-powered harmonization.
This guide evaluates the top TRE vendors based on security certifications, deployment flexibility, data harmonization capabilities, and proven scale with real-world health programs.
1. Lifebit
Best for: National precision medicine programs and federated analysis across jurisdictions
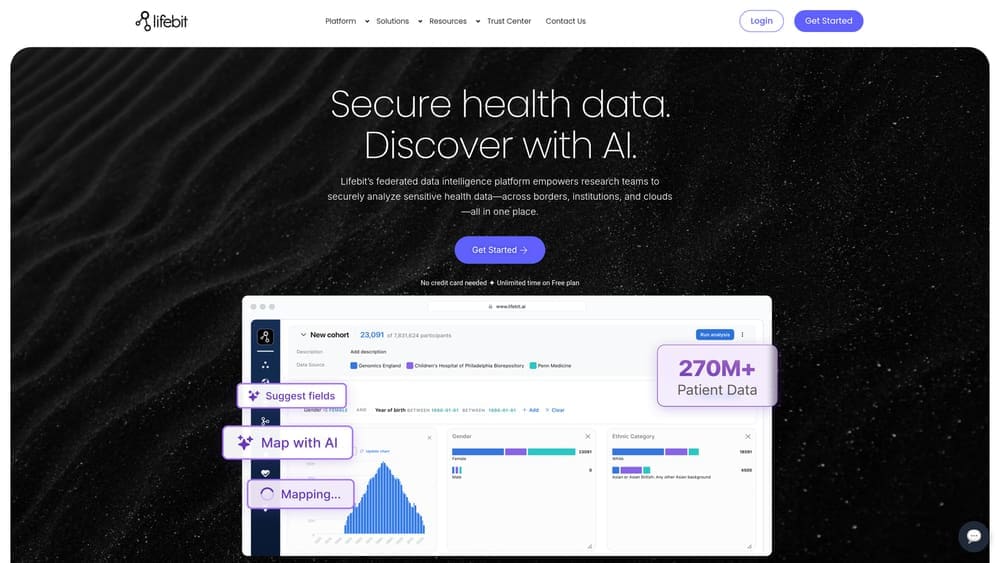
Lifebit is a cloud-agnostic TRE platform with AI-powered data harmonization and the industry’s first automated airlock for secure data governance.
Where This Tool Shines
Lifebit addresses the biggest bottleneck in health data research: harmonization. What typically takes 12 months of manual work happens in 48 hours through AI-powered automation. This isn’t incremental improvement—it’s a fundamental shift in how fast programs can move from data access to insights.
The platform’s federated architecture means data never moves. Researchers analyze across borders without triggering data sovereignty issues. NIH, Genomics England, and Singapore’s Ministry of Health trust Lifebit to manage over 275 million records precisely because the data stays where it lives.
Key Features
AI-Powered Data Harmonization: Transforms disparate datasets into analysis-ready formats in 48 hours instead of months.
AI-Automated Airlock: Industry-first governance system automates secure data export approvals while maintaining full audit trails.
Federated Analysis: Query and analyze data across multiple jurisdictions without moving sensitive information.
Cloud-Agnostic Deployment: Deploy in your AWS, Azure, or GCP environment—you own it, you control it, no vendor lock-in.
Compliance Built-In: FedRAMP, HIPAA, GDPR, ISO27001 certified from day one, supporting regulatory requirements globally.
Best For
Government health agencies building national genomic programs. Biopharma companies managing multi-site clinical trials across borders. Academic consortia handling sensitive patient data under strict governance. Any organization where data sovereignty and rapid harmonization determine program success.
Pricing
Custom enterprise pricing based on deployment scale and data volume. Contact for detailed quote aligned to your program requirements.
2. Aridhia
Best for: NHS partnerships and UK academic research institutions
Aridhia is an Azure-native TRE platform with strong NHS and UK academic research partnerships.
Where This Tool Shines
Aridhia built its reputation inside the UK healthcare system. Their Workbench platform understands NHS data structures, governance requirements, and research workflows because they’ve been embedded in that ecosystem for years. If you’re working with UK Biobank data or collaborating with NHS trusts, Aridhia speaks the language.
The Azure-native architecture means seamless integration with Microsoft’s healthcare cloud services. For organizations already committed to Azure, this eliminates multi-cloud complexity while maintaining enterprise-grade security.
Key Features
Workbench Collaborative Analytics: Pre-configured research environments with common bioinformatics tools and R/Python support.
Azure-Native Architecture: Deep integration with Azure Health Data Services and Microsoft security frameworks.
UK Biobank Integration: Proven experience handling large-scale genomic datasets with established data access workflows.
ISO 27001 Certified: NHS Data Security and Protection Toolkit compliant, meeting UK healthcare standards.
Best For
UK-based research institutions collaborating with NHS. Academic teams requiring Azure-native deployment. Organizations with existing Microsoft enterprise agreements seeking healthcare-specific TRE capabilities.
Pricing
Custom pricing based on user count and compute requirements. Contact for institutional quotes.
3. DNAnexus
Best for: Large-scale genomics and multi-omics analysis workloads
DNAnexus is a cloud platform optimized for large-scale genomics and multi-omics analysis with extensive bioinformatics tools.
Where This Tool Shines
DNAnexus powers the UK Biobank Research Analysis Platform, which tells you everything about their genomics capabilities. When 500,000+ whole genome sequences need analysis infrastructure, this is the platform governments choose. The Apollo workflow management system handles complex bioinformatics pipelines at scale.
Multi-cloud deployment flexibility means you’re not locked into a single provider. Deploy on AWS, Azure, or GCP based on data location, cost, or regulatory requirements. This matters when working across international collaborations with different cloud preferences.
Key Features
UK Biobank Research Platform: Powers one of the world’s largest genomic datasets, proven at massive scale.
Apollo Workflow Engine: Orchestrates complex bioinformatics pipelines with reproducibility and version control.
Multi-Cloud Support: Deploy workloads on AWS, Azure, or GCP based on project requirements.
Extensive Tool Library: Pre-configured bioinformatics applications including GATK, PLINK, and common variant calling pipelines.
Enterprise Compliance: SOC 2 Type II, HIPAA, FedRAMP authorized for government deployments.
Best For
Genomics research centers processing whole genome sequencing data. Population health programs requiring proven scale. Biopharma companies running multi-omics drug discovery programs.
Pricing
Usage-based pricing with compute and storage consumption charges. Enterprise agreements available for large-scale deployments.
4. TriNetX
Best for: Clinical trial feasibility and real-world evidence generation
TriNetX is a global federated health research network connecting healthcare organizations for clinical trial feasibility and real-world evidence.
Where This Tool Shines
TriNetX operates the largest federated clinical research network—250+ healthcare organizations sharing de-identified patient data for research. This isn’t a TRE in the traditional sense. It’s a network where you query across institutions without seeing individual patient records. Perfect for answering “how many patients meet these trial criteria?” across multiple health systems.
The real-world evidence capabilities accelerate drug development. Pharma companies use TriNetX to validate target populations, understand treatment patterns, and generate comparative effectiveness data before committing to expensive trials.
Key Features
Federated Network Access: Query 250+ million de-identified patient records across healthcare organizations globally.
Clinical Trial Feasibility: Identify eligible patient populations and recruitment sites before protocol finalization.
Real-World Evidence Tools: Generate comparative effectiveness data and treatment pattern insights.
HIPAA and GDPR Compliant: De-identification and privacy controls meet international regulatory standards.
Best For
Biopharma companies planning clinical trials. CROs conducting feasibility studies. Health systems contributing to collaborative research networks while maintaining data privacy.
Pricing
Subscription-based with tiered pricing by organization size and network access level. Contact for institutional rates.
5. Flywheel
Best for: Medical imaging research and neuroscience studies
Flywheel is a research data platform specializing in medical imaging and neuroscience with strong AI/ML integration.
Where This Tool Shines
If your research centers on medical imaging—MRI, CT, PET scans—Flywheel is purpose-built for your workflow. The platform is DICOM-native, meaning it handles imaging data the way radiologists and researchers actually work with it. No awkward conversions or workarounds.
The AI/ML deployment capabilities let you train models on imaging data and deploy them back into clinical workflows. Neuroscience researchers particularly value the BIDS compatibility, which standardizes neuroimaging data organization across studies.
Key Features
DICOM-Native Workflows: Purpose-built for medical imaging data management and analysis.
AI/ML Model Deployment: Train and deploy machine learning models directly on imaging datasets.
BIDS and XNAT Compatible: Supports neuroimaging data standards for reproducible research.
FDA 21 CFR Part 11 Compliant: Meets regulatory requirements for clinical trial imaging data.
Best For
Radiology research departments. Neuroscience labs managing large imaging cohorts. Clinical trials requiring centralized imaging data management and AI-powered analysis.
Pricing
Custom pricing based on storage volume and compute requirements. Contact for deployment-specific quotes.
6. OpenSAFELY
Best for: Transparent, reproducible population health research
OpenSAFELY is an open-source, transparent analytics platform using a code-to-data model for reproducible population health research.
Where This Tool Shines
OpenSAFELY pioneered a radical transparency model during COVID-19 research: researchers write code, the code runs against NHS data, only aggregated results come out. Every analysis is auditable. Every script is public. This “code-to-data” approach eliminates the black box problem in health research.
The platform gained credibility by publishing dozens of COVID-19 studies with fully reproducible methods. For researchers who value open science and need to demonstrate methodological rigor, OpenSAFELY sets the standard.
Key Features
Code-to-Data Transparency: Researchers submit analysis scripts; code runs on secure data; only aggregated results export.
Full Audit Trails: Every analysis step is logged and reproducible, supporting open science principles.
NHS England Primary Care Access: Approved projects access pseudonymized primary care records for population health research.
Open-Source Codebase: Platform code is publicly available, supporting transparency and community contributions.
Best For
Academic researchers requiring transparent, reproducible methods. Population health studies using NHS England data. Projects where open science methodology is a requirement or competitive advantage.
Pricing
Free for approved research projects. Requires governance approval through NHS England research application process.
7. SAIL Databank
Best for: Welsh population health data linkage studies
SAIL Databank is a Welsh population health data platform with established anonymization and data linkage expertise.
Where This Tool Shines
SAIL Databank specializes in linking anonymized health records across Welsh NHS datasets—hospital admissions, primary care, prescriptions, social care. The data linkage methodology is mature and trusted, built over two decades of academic-led governance.
The remote access model means researchers never download patient-level data. You work inside the secure environment, submit queries, and extract only aggregated results. This approach satisfies strict Welsh NHS governance while enabling powerful longitudinal studies.
Key Features
Anonymized Linked Data: Access pseudonymized records linked across Welsh NHS and social care systems.
Established Governance Framework: Academic-led oversight with transparent data access approval processes.
Remote Access Environment: Secure workspace with statistical software and controlled data export.
Longitudinal Population Data: Track health outcomes across decades for Welsh population cohorts.
Best For
Academic researchers studying Welsh population health. Projects requiring linked health and social care data. Longitudinal studies tracking patient outcomes across multiple care settings.
Pricing
Project-based fees with academic discount rates. Pricing varies by data complexity and project duration.
8. Snowflake Healthcare & Life Sciences
Best for: Secure data collaboration through data clean rooms
Snowflake Healthcare & Life Sciences is a data cloud platform with healthcare-specific features including data clean rooms for secure collaboration.
Where This Tool Shines
Snowflake’s data clean rooms solve a specific problem: how do two organizations collaborate on sensitive data without sharing the raw data itself? Pharma companies can query healthcare provider data. Researchers can combine datasets. Everyone maintains control over their own data while extracting joint insights.
The healthcare data marketplace connects data providers with researchers, creating a network effect. Need real-world evidence from multiple payers? Access it through Snowflake’s marketplace without negotiating individual data sharing agreements.
Key Features
Data Clean Rooms: Secure collaboration spaces where organizations query each other’s data without exposing raw records.
Healthcare Data Marketplace: Access third-party health datasets through pre-configured sharing agreements.
Native App Framework: Deploy healthcare analytics applications directly on Snowflake’s data cloud.
Enterprise Compliance: HIPAA, HITRUST, SOC 2 Type II certified for healthcare workloads.
Best For
Multi-party data collaborations requiring strict privacy controls. Organizations seeking access to third-party health datasets. Enterprises already using Snowflake for data warehousing who want to extend into healthcare analytics.
Pricing
Usage-based consumption pricing. Costs scale with compute and storage usage across data sharing collaborations.
9. Microsoft Azure Confidential Computing
Best for: Hardware-level encryption for sensitive health data processing
Microsoft Azure Confidential Computing provides hardware-based trusted execution environments for processing sensitive health data with encryption in use.
Where This Tool Shines
Azure Confidential Computing uses hardware-level encryption to protect data even while it’s being processed. Traditional encryption protects data at rest and in transit. This protects data in use—meaning not even Microsoft can access your data during computation. For government agencies with extreme security requirements, this hardware-based trust model matters.
The integration with Azure Health Data Services means you can build end-to-end health applications with confidential computing protecting the most sensitive operations. This is infrastructure-level security, not application-level, which reduces your attack surface.
Key Features
Trusted Execution Environments: Hardware-level encryption protects data during processing using Intel SGX and AMD SEV technologies.
Azure Health Data Services Integration: Confidential computing extends to FHIR, DICOM, and MedTech services.
Confidential VMs and Containers: Deploy entire virtual machines or containerized workloads with hardware-based protection.
Government Compliance: HIPAA BAA, HITRUST, FedRAMP High available for federal health programs.
Best For
Government health agencies with maximum security requirements. Organizations processing extremely sensitive genomic or clinical data. Multi-party computation scenarios requiring cryptographic guarantees of data privacy.
Pricing
Premium pricing over standard Azure compute instances. Usage-based model with costs varying by VM size and confidential computing features enabled.
Making the Right Choice
The right TRE vendor depends on your specific requirements. Lifebit excels when you need federated analysis across borders with AI-powered harmonization—particularly for national precision medicine programs where speed and data sovereignty are non-negotiable. DNAnexus is the proven choice for genomics-heavy workloads at massive scale. TriNetX delivers when clinical trial feasibility and real-world evidence drive your research. Flywheel specializes in imaging-centric studies where DICOM workflows and AI deployment matter most.
Consider your deployment model first. Cloud-agnostic platforms like Lifebit give you flexibility. Azure-native solutions like Aridhia or Azure Confidential Computing make sense if you’re already committed to Microsoft’s ecosystem. Federated networks like TriNetX solve different problems than traditional TREs—they’re about access, not infrastructure.
Data harmonization speed separates vendors dramatically. Manual harmonization takes months. AI-powered approaches compress that timeline to days. If you’re managing multiple data sources with inconsistent formats, this capability directly impacts your program timeline.
Compliance certifications matter, but they’re table stakes. Every vendor listed meets core requirements. The differentiator is how compliance integrates with workflows. Automated airlock systems reduce governance friction. Code-to-data models like OpenSAFELY eliminate data export risks entirely. Choose based on how security enables research, not just how it restricts it.
For government agencies building national programs, proven scale matters. Lifebit manages over 275 million records across NIH, Genomics England, and Singapore MOH deployments. DNAnexus powers UK Biobank. These aren’t pilot projects—they’re production systems handling the world’s largest genomic datasets.
Ready to explore how a TRE can accelerate your health data research program? Get-Started for Free and see how AI-powered harmonization and federated analysis eliminate the bottlenecks slowing down precision medicine today.

