Beyond the Clinic: Exploring Decentralized Clinical Analytics and Digital Health

How Decentralised Clinical Analytics Delivers a 13x ROI in Phase III Trials
Decentralised clinical analytics refers to the use of remote technologies, federated data architectures, and digital health tools to conduct clinical research outside traditional site-based settings—allowing patients to participate from home while enabling real-time data analysis across distributed sources without moving sensitive information. This paradigm shift moves the locus of the trial from the academic medical center to the patient’s daily environment, utilizing a “hub-and-spoke” model where the central investigator maintains oversight through a digital nervous system of connected devices and platforms.
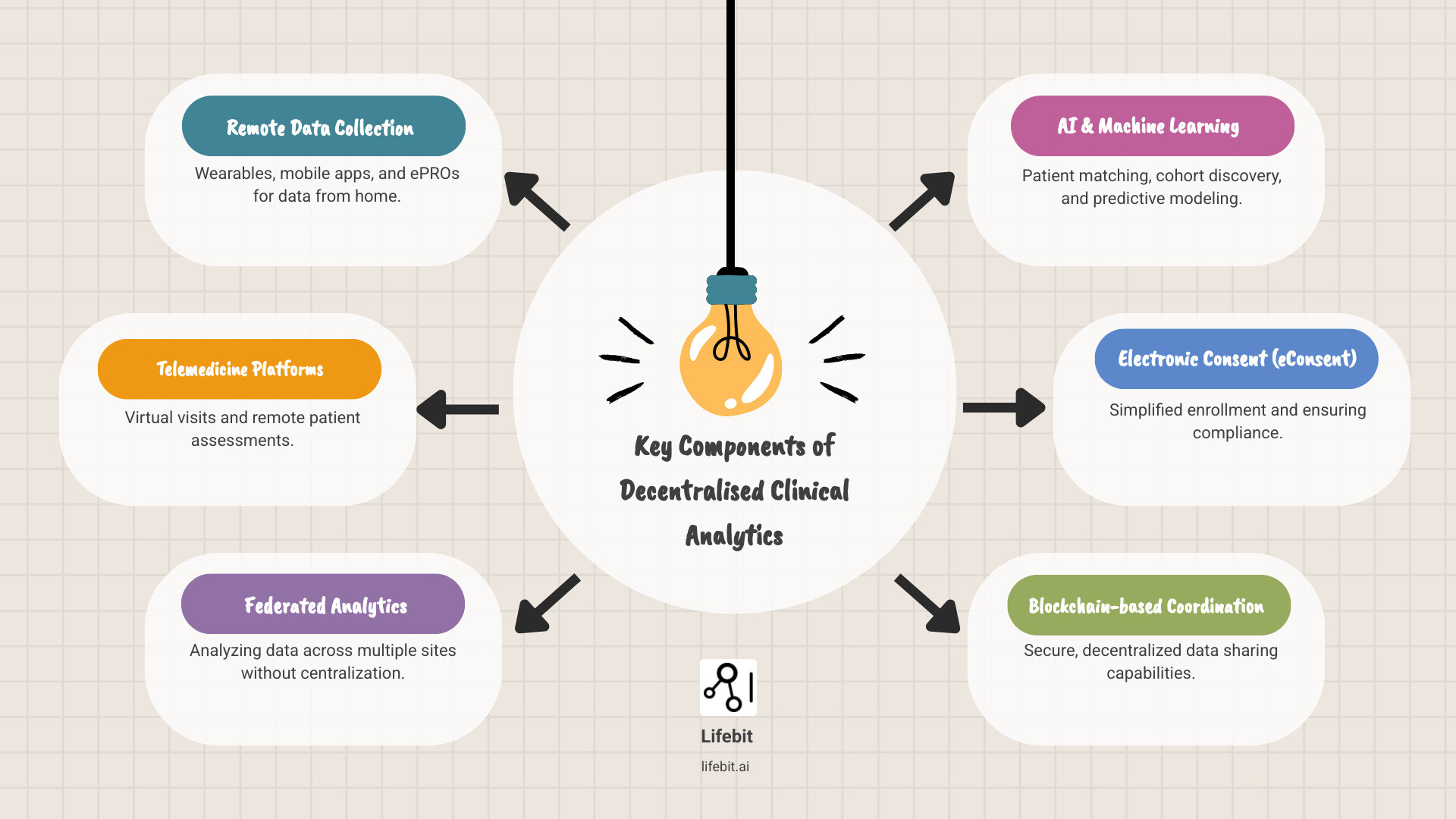
Key components of decentralised clinical analytics include:
- Remote data collection through medical-grade wearables, mobile apps, and electronic patient-reported outcomes (ePRO) that capture high-frequency physiological data.
- Telemedicine platforms for virtual visits, remote physical assessments, and real-time adverse event monitoring.
- Federated analytics that analyze data across multiple sites, jurisdictions, and hospital systems without the need for physical data centralization, preserving privacy and data sovereignty.
- AI and machine learning for automated patient matching, cohort discovery, and predictive modeling to identify potential dropouts before they occur.
- Electronic consent (eConsent) to simplify enrollment, ensure regulatory compliance through immutable audit trails, and improve participant comprehension via multimedia modules.
- Blockchain-based coordination for secure, decentralized data sharing and transparent tracking of biological samples and drug shipments.
The promise seemed enormous. Academic models, most notably those developed by the Tufts Center for the Study of Drug Development (CSDD), predicted a 13-fold return on investment in Phase III trials. This ROI is not merely a theoretical projection; it is rooted in the reduction of cycle times, the avoidance of costly protocol amendments, and the mitigation of recruitment failures. The COVID-19 pandemic acted as a global catalyst, forcing the industry to adopt remote monitoring at scale. By 2021, over 1,200 trials incorporated decentralized elements—a 50% jump from 2019.
But then something unexpected happened. DCT numbers declined after 2021, falling back toward pre-pandemic levels in terms of “fully” decentralized models. Despite regulatory support from the FDA and growing interest from sponsors, only 2.6% of trials adopted fully decentralized models. Most stuck with hybrid approaches—97.6% used partial decentralization, primarily remote data collection, while only 4.1% implemented remote recruitment. This suggests that while the industry values the data, it remains hesitant to abandon the safety net of the physical site.
The gap between expectations and reality reveals a deeper challenge: decentralized clinical analytics requires more than just technology. It demands new workflows, sophisticated statistical methods to handle missing data, regulatory coordination across borders, and federated infrastructure that can handle diverse data types—from high-depth genomics to longitudinal imaging to real-world evidence—without compromising privacy or quality.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where I’ve spent over 15 years building platforms that enable secure decentralised clinical analytics across federated biomedical data networks. My work has powered COVID-19 research, rare disease studies, and precision medicine initiatives for public sector institutions and pharmaceutical organizations worldwide. I have seen firsthand how the transition from “data silos” to “federated insights” can shave years off drug development timelines, and I’ve seen what it actually takes to make decentralized trials deliver on their promise.
Decentralised clinical analytics basics:
The Core Technologies Powering Decentralised Clinical Analytics
To move research beyond the four walls of a hospital, we need a robust digital backbone. The shift toward decentralised clinical analytics is driven by a suite of Digital Health Technologies (DHTs) that bridge the gap between a patient’s living room and the researcher’s dashboard. This is not just about replacing a paper form with a digital one; it is about re-engineering the data flow of clinical science.
At the heart of this transition is Electronic Consent (eConsent). Traditionally, signing up for a trial involved thick stacks of paper and a physical trip to a clinic, often leading to “signature fatigue” where patients sign without full comprehension. eConsent uses interactive multimedia tools, knowledge checks, and electronic signatures to ensure participants truly understand the study risks from the comfort of their homes. This digital-first approach also allows for real-time updates; if a protocol changes, investigators can re-consent thousands of patients instantly via their mobile devices.
Once enrolled, Telemedicine becomes the primary vehicle for interaction, replacing routine check-ups with virtual consultations. However, the real power lies in data integration. By leveraging Electronic Health Records (EHRs) and wearable sensors, researchers can capture “continuous” data rather than “snapshot” data. Instead of measuring blood pressure once a month at a clinic—where “white coat hypertension” often skews results—we can monitor it every hour in the patient’s natural environment. This Technology for successful decentralised clinical trials ensures that the data being analyzed is a true reflection of the patient’s daily life, providing a much higher resolution of the drug’s efficacy and safety profile.
AI and Machine Learning in Decentralised Clinical Analytics
When data is scattered across thousands of homes and hundreds of local clinics, traditional centralized AI models break down. You cannot simply “upload” petabytes of sensitive genomic or imaging data to a single cloud without hitting massive privacy, security, and bandwidth walls. The “Data Gravity” problem means that as datasets grow larger, they become harder to move.
This is where Swarm Learning and federated AI come into play. As detailed in the research on Swarm Learning for decentralized and confidential clinical machine learning, this technology allows AI models to “travel” to the data. The data stays securely behind local firewalls (e.g., within a hospital’s or a patient’s local node), while the AI learns from it and shares only the “insights”—the weights of the neural network—with a central coordinator. This ensures that no personal identifiable information (PII) ever leaves its original jurisdiction.
We are also seeing the rise of Digital Twins—virtual models of patients built from their real-time data and historical health records. These twins allow us to simulate trial outcomes before a single drug is administered, significantly reducing risk and cost. For instance, in 2018, advanced predictive matching at the Mayo Clinic increased breast cancer patient enrollment by 80% in just 11 months by using AI to scan EHRs and match patients to decentralized trial protocols that they could actually complete.
Real-World Evidence and Federated Data Architectures
The “Holy Grail” of modern research is the seamless integration of Decentralized Clinical Trials in the Era of Real‐World Evidence. Clinical trials have historically been “clean” but “artificial,” conducted on highly curated populations that don’t always reflect the general public. Real-world evidence (RWE) is “messy” but “true,” reflecting how drugs perform in the wild.
Decentralised clinical analytics allows us to marry these two worlds. By using federated data architectures, we can query multi-omic data, pharmacy records, and insurance claims across different geographies without moving the underlying records. This solves the problem of data silos—where life-saving information is trapped in incompatible systems—and ensures interoperability across the global research community. By applying the OMOP Common Data Model (CDM) to these federated sources, researchers can run the same analytical script across fifty different global databases simultaneously, achieving a scale of evidence that was previously unimaginable.
Why Sponsors See a 13x ROI with Decentralized Models
The financial argument for decentralised clinical analytics is staggering. While the initial setup costs for DHTs and federated infrastructure can be high, the long-term savings are driven by three primary factors: speed of recruitment, patient retention, and the reduction of site-related overhead.
An economic model published in Therapeutic Innovation & Regulatory Science found that applying decentralized methods results in a five-fold return on investment (ROI) in Phase II trials and an incredible 13-fold ROI in Phase III. Why is the Phase III impact so much higher? Because Phase III trials are the largest and most expensive part of the drug development lifecycle. The biggest cost in any trial is time. A single day of delay in bringing a blockbuster drug to market can cost a sponsor between $600,000 and $8 million in lost patent life and potential revenue.
Decentralized models tackle the “recruitment bottleneck” head-on. Historically, 80% of clinical trials fail to meet their enrollment timelines, and 50% of sites enroll one or zero patients. In 2016, decentralized search tools were used to find qualified participants for a cardiac trial in minutes—a process that would have taken human reviewers six months of manual chart review. Furthermore, by reducing the “travel burden” on patients, these trials see significantly higher retention rates. When participation is as easy as opening an app or visiting a local pharmacy for a blood draw, patients are far less likely to drop out due to the logistical exhaustion of traveling to a distant academic center.
Improving Equity and Representation through Decentralised Clinical Analytics
Perhaps the most vital benefit of decentralised clinical analytics is its ability to fix the “diversity gap” in medical research. Traditional trials are often limited to people who live near major academic medical centers in wealthy, urban areas. This creates a “postcode lottery” for life-saving treatments and results in clinical data that is overwhelmingly skewed toward Caucasian populations. This lack of diversity is not just a social issue; it is a scientific one, as different ethnic groups may respond differently to medications due to genetic variations.
By moving “beyond the clinic,” we can reach underserved populations, rural communities, and those with limited mobility. However, we must be careful not to trade a “geographic divide” for a “digital divide.” Research into Decentralized clinical trials as a new paradigm of trial delivery to improve equity of access suggests that we must provide multilingual support, provide hardware to participants who don’t own smartphones, and utilize community-based hubs (like local pharmacies) to ensure that those without high-speed internet aren’t left behind. True equity in decentralization means meeting the patient where they are, both physically and technologically.
Therapeutic Applications in Oncology and Rare Diseases
In certain fields, decentralised clinical analytics isn’t just a “nice to have”—it’s a necessity for the trial to even exist.
- Rare Diseases: With only a handful of patients scattered across the globe, a site-based trial is often physically impossible. Decentralization allows for remote recruitment across continents and direct-to-patient drug delivery, enabling research into conditions that were previously ignored by the industry.
- Oncology: Cancer patients are often immunocompromised or too ill to travel frequently. The FDA’s Real-Time Oncology Review (RTOR) pilot program is exploring how decentralized data can accelerate the approval of promising therapies. By reviewing efficacy data the moment it is generated by a wearable or a local lab, regulators can make decisions in weeks rather than months, which is a literal life-saver for patients with late-stage disease.
Navigating the Post-Pandemic Reality and Regulatory Problems
If the benefits are so clear, why did DCT adoption decline after 2021? The answer lies in the “complexity of reality.” During the pandemic, regulators granted emergency flexibility because the alternative was the total collapse of clinical research. As the world returned to “normal,” the industry had to reconcile these new, agile methods with long-standing safety, data integrity, and “Chain of Custody” laws.
The FDA has been a proactive leader here, issuing a landmark draft guidance in May 2023 on how to conduct a DCT. This guidance clarifies how to maintain patient safety and data quality when the investigator isn’t in the same room as the participant. It emphasizes that while tasks can be delegated to local healthcare providers, the Principal Investigator (PI) remains ultimately responsible for the trial’s conduct. Similarly, the International Council for Harmonisation (ICH) is updating its Good Clinical Practice (GCP) E6(R3) guidelines to reflect the digital age, focusing on “Quality by Design” (QbD) principles where digital risks are identified and mitigated before the trial begins.
Addressing Data Integrity and Statistical Challenges in Decentralised Clinical Analytics
One of the biggest problems in decentralised clinical analytics is “data noise.” When a patient takes their own blood pressure at home, is it as accurate as a nurse doing it in a clinic? What happens if a patient forgets to wear their sensor for two days?
Statistical researchers are using the Estimand Framework to account for these variables. As discussed in Scientific considerations for DCTs, we must use sensitivity analysis and stratified modeling to handle “intercurrent events”—like a patient’s wearable device running out of battery or a technical failure during a data upload. Advanced imputation methods are now being used to fill in these “data gaps” without introducing bias, ensuring that the final analysis remains robust enough for regulatory submission. The goal is to move from “perfect data on a few people” to “representative data on many people.”
Overcoming Regional Discoordination and Privacy Laws
Global trials face a patchwork of conflicting regulations that can stifle innovation. While the US might allow certain remote data practices, the EU’s GDPR or Singapore’s PDPA might have much stricter requirements for cross-border data sharing. The legal definition of “anonymized data” varies significantly between jurisdictions, making it difficult to create a single global dataset.
The recent Executive order on preventing access to Americans’ bulk sensitive personal data adds another layer of complexity, particularly for trials involving genomic data. To succeed, sponsors must use federated platforms—like Lifebit—that allow for “local” compliance. By keeping data within its country of origin while still allowing for global analysis via federated queries, we can respect national sovereignty and privacy laws without stopping scientific progress. This “data-resident” approach is becoming the standard for international pharmaceutical collaboration.
Frequently Asked Questions about Decentralized Trials
What are the primary benefits of adopting DCTs for patients?
The primary benefit is reduced participant burden. Patients no longer need to take time off work, arrange childcare, or spend hours traveling to a clinic. It also allows for continuous monitoring, meaning potential safety issues or adverse reactions can be spotted in real-time by AI algorithms rather than waiting for the next scheduled appointment. Finally, it provides access to cutting-edge treatments for patients living in rural or underserved areas who would otherwise be excluded from clinical research.
How does the FDA guide the implementation of decentralized elements?
The FDA issued a comprehensive draft guidance in May 2023. Key recommendations include ensuring that the Principal Investigator (PI) maintains active oversight even when using local healthcare providers, ensuring that Digital Health Technologies (DHTs) are “fit-for-purpose” (meaning they are validated for the specific population being studied), and having a clear, documented plan for managing adverse events that occur remotely. They also established a Digital Health Advisory Committee to help the agency stay ahead of technological shifts like generative AI and remote sensing.
Why has there been a decline in DCT adoption since 2021?
The decline is largely due to the “hybrid middle ground.” While 97.6% of trials use some digital elements, very few are fully decentralized. The industry is currently in a “stabilization phase,” where sponsors are moving away from emergency pandemic measures and toward more sustainable, long-term digital strategies. High start-up costs, the need for specialized staff to manage remote tech, and a lack of standardized global regulations also remain significant barriers that the industry is working to overcome.
Is the data from decentralized trials as reliable as traditional site-based data?
Yes, provided the trial is designed with “Quality by Design” principles. In many cases, decentralized data is more reliable because it captures a larger volume of real-world data points, reducing the impact of outliers and “white coat” effects. Regulators like the FDA and EMA accept decentralized data as long as the sponsor can demonstrate a clear audit trail and prove that the DHTs used were properly calibrated and validated.
What role does Federated Learning play in these trials?
Federated Learning allows researchers to train AI models on data located at different sites without actually moving the data. This is crucial for maintaining patient privacy and complying with strict data protection laws like GDPR. It allows for the analysis of massive, diverse datasets (like genomics) that are too large or sensitive to be centralized in a single cloud repository.
Conclusion: The Era of Living Protocols
The future of decentralised clinical analytics isn’t about replacing the doctor; it’s about empowering the patient and the researcher with better, more frequent, and more representative data. We are moving toward an era of “Living Protocols”—trials that are no longer static documents but dynamic systems that can adapt in real-time based on the data flowing in from thousands of remote nodes.
This shift requires a fundamental change in how we think about clinical evidence. We must move away from the idea that data is only valid if it is collected within the sterile environment of a hospital. Instead, we must embrace the complexity of the real world, using federated AI and robust statistical frameworks to extract truth from the noise of daily life.
At Lifebit, we believe that the only way to realize the 13x ROI and the promise of global equity is through federated analytics. By connecting the world’s biomedical data without moving it, we can ensure that every patient, regardless of where they live or their socioeconomic status, can contribute to and benefit from the next generation of medical breakthroughs. The clinic is no longer a building; it is a connection. And that connection is powered by analytics.

