Beyond the Hype Making Sense of Real-World Data in Healthcare

Why Real-World Data Analytics Matters for Modern Healthcare
Real-world data analytics in healthcare transforms how we understand patient outcomes, accelerate drug development, and improve care delivery. Unlike traditional clinical trials that operate in controlled settings, real-world data (RWD) captures what actually happens when patients receive treatment in everyday medical practice—from electronic health records and insurance claims to wearable devices and patient registries.
Key components of real-world data analytics:
- Data Sources – Electronic health records (EHRs), medical claims, pharmacy databases, patient registries, wearables, and home monitoring devices
- Analytics Methods – Statistical analysis, machine learning, deep learning, and causal inference techniques
- Applications – Drug safety surveillance, treatment effectiveness studies, clinical trial design, market access decisions, and precision medicine
- Benefits – Faster drug approvals, reduced development costs (potentially saving hundreds of millions of dollars), improved patient targeting, and improved post-market safety monitoring
- Challenges – Data quality issues, privacy regulations, lack of standardization, and need for specialized analytics expertise
The promise is substantial. Patient-level RWD can save drug developers hundreds of millions of dollars by accelerating trials, expanding approvals, and optimizing pricing models. It enables pharmaceutical companies to track real patient journeys, identify safety concerns early, and demonstrate value to regulators and payers—all while capturing populations often excluded from traditional trials.
But the reality is messy. Most healthcare organizations struggle to transform massive volumes of unstructured, fragmented data into actionable insights. The data sits in silos across different systems, uses inconsistent formats, and requires sophisticated analytics expertise to extract meaningful patterns. Privacy regulations like GDPR and HIPAA add complexity. And without proper validation, RWD can introduce biases that undermine decision-making.
The gap between potential and practice is where innovation happens. Regulatory bodies like the FDA now explicitly recognize RWD’s role in supporting drug approvals through the 21st Century Cures Act. Advanced analytics platforms using AI and machine learning can now process multi-terabyte datasets at scale. Standardization efforts through frameworks like OHDSI/OMOP enable cross-institutional research. And federated approaches allow analysis without moving sensitive patient data.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent years building federated platforms that make real-world data analytics in healthcare both secure and scalable for pharma companies, public health agencies, and research institutions worldwide. This guide cuts through the hype to show you what actually works—from data sources and analytics methods to regulatory requirements and implementation strategies.
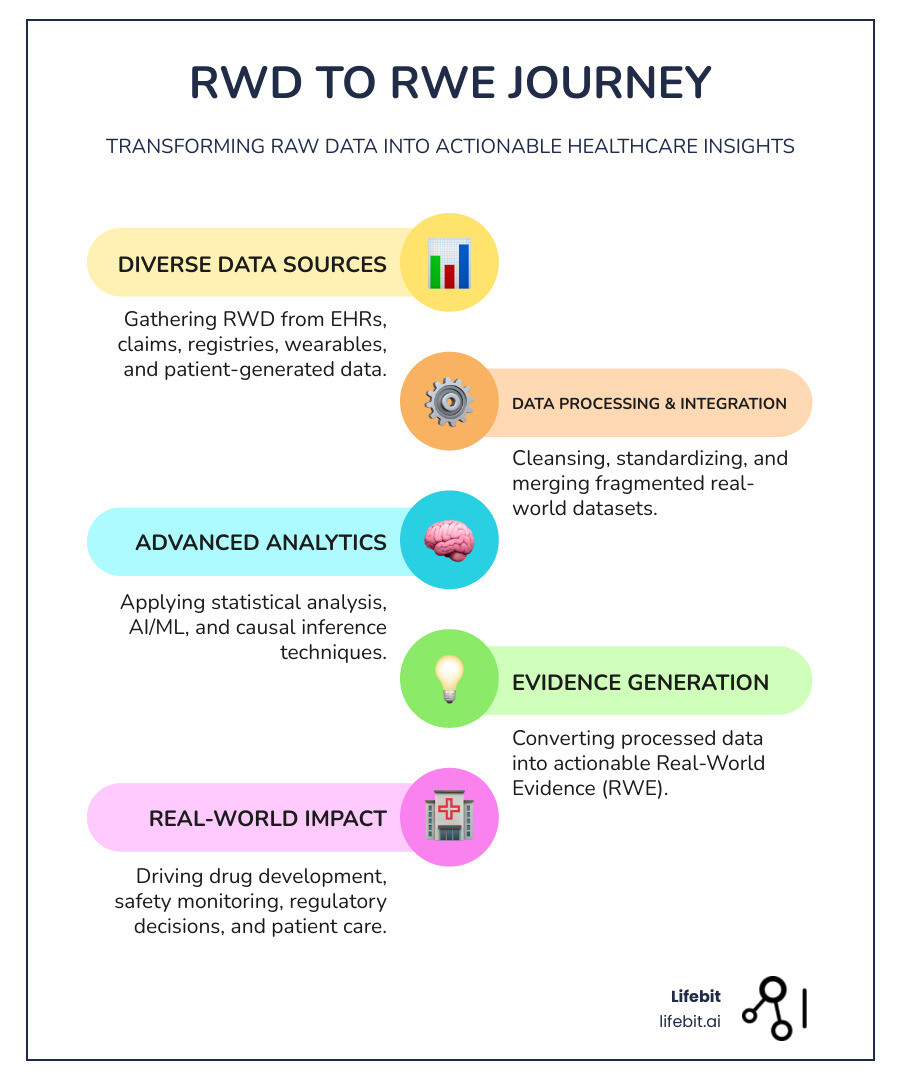
Real-world data analytics in healthcare terms simplified:
Real-World Data Analytics in Healthcare: From Raw Data to Patient Insights
The journey from a patient’s doctor visit to a life-saving medical insight is paved with data. Every interaction in the healthcare system—a prescription filled, a diagnostic test run, or even a heart rate logged on a smartwatch—contributes to a massive, growing ocean of information. However, raw data by itself is just noise. To find the signal, we need real-world data analytics in healthcare.
Bridging the Efficacy-Effectiveness Gap
Traditional clinical trials are the “gold standard” for a reason: they are controlled. But that control is also a limitation. Trials often exclude pregnant women, the elderly, or patients with multiple chronic conditions to keep variables simple. This creates a gap between “efficacy” (how a drug works in a perfect, controlled environment) and “effectiveness” (how it works in the general population). RWD is “dirty” but honest. It shows us how a drug performs in a 75-year-old with three other comorbidities who might forget a dose now and then. By analyzing these real-world scenarios, researchers can identify sub-populations where a drug might be more or less effective, leading to more personalized treatment protocols.
Primary Sources of RWD
To build a complete picture of the patient journey, we must look across several distinct data silos. In our work at Lifebit, we see how integrating these sources creates a longitudinal view of health that was previously impossible.
- Electronic Health Records (EHRs): These contain the “clinical truth”—physician notes, lab results, and imaging. While rich, EHR data is often unstructured. For example, a doctor’s note might mention a patient’s “fatigue” or “social isolation,” which are critical data points that don’t always appear in standardized billing codes. Extracting this requires advanced Natural Language Processing (NLP).
- Insurance Claims and Billing Data: This is a powerful source for tracking high-level patient movement. Databases like the MarketScan Medicare Supplemental Database integrate inpatient and outpatient claims with drug and lab data, covering millions of lives. This data is excellent for understanding healthcare utilization and costs, though it lacks the clinical depth of EHRs.
- Product and Disease Registries: These are gold mines for rare diseases. Because rare disease populations are small, registries provide the robust evidence needed for regulatory-grade analysis when traditional trials aren’t feasible. They often track patients over decades, providing invaluable long-term safety data.
- Patient-Generated Data and Wearables: From smartwatches to home health monitors, this data provides a 24/7 look at patient health status outside the clinic. This “continuous monitoring” can detect arrhythmias or respiratory changes long before a patient schedules a doctor’s visit.
- Pharmacy Databases: These track medication adherence and longitudinal prescription (LRx) patterns. They reveal the “persistence” of a treatment—how long a patient stays on a drug before switching or stopping, which is a key indicator of real-world tolerability.
Changing RWD into Actionable Evidence
The magic happens when we transform Real-World Data (RWD) into Real-World Evidence (RWE). This isn’t just a name change; it’s a rigorous scientific process involving several critical steps:
- Data Cleansing and De-duplication: Real-world data is notoriously “dirty.” We have to solve the “merge/purge” problem—ensuring that a patient record in an EHR matches the same patient in a claims database without compromising their privacy. This often involves sophisticated probabilistic matching algorithms.
- Standardization and Normalization: We use common data models like OHDSI/OMOP to ensure that “diabetes” in a New York hospital is coded the same way as “diabetes” in a London clinic. This allows for cross-border studies that increase the statistical power of the analysis.
- Veracity and Quality Assessment: We must assess the quality of the data. Is it complete? Is it traceable? Regulatory bodies like the FDA require high standards of reliability, including an audit trail of how the data was handled, before they will accept RWE for decision-making.
By synthesizing these sources, we get a longitudinal view of the patient. We move from seeing a single “data point” to seeing a whole “patient journey,” allowing for a more holistic understanding of disease progression and treatment impact.
For more on these methods, you can explore this comprehensive review of RWD methods and applications.
Leveraging AI and Machine Learning for Advanced Analytics
As the volume of healthcare data reaches petabyte scale, humans simply cannot keep up. This is where AI and machine learning (ML) become the engines of real-world data analytics in healthcare. These tools don’t just process data faster; they find patterns that are invisible to the naked eye and allow for the analysis of multi-modal datasets that combine genomics, imaging, and clinical records.
Leveraging AI for Real-World Data Analytics in Healthcare
AI allows us to move beyond descriptive analytics (“what happened”) to predictive and prescriptive analytics (“what will happen” and “how can we fix it”).
- Pattern Recognition and Phenotyping: ML algorithms can scan millions of records to identify “probabilistic phenotypes”—patients who likely have a condition like type 2 diabetes or a rare autoimmune disorder even if it hasn’t been formally coded yet. This is crucial for early intervention and clinical trial recruitment.
- Natural Language Processing (NLP): Much of the best clinical data is trapped in unstructured doctor’s notes. NLP “reads” these notes to extract symptoms, social determinants of health (like housing stability or diet), and adverse reactions that might not be captured in structured fields.
- Deep Learning in Imaging: AI is now used to segment COVID-19 pneumonia in CT scans or classify lung patterns in interstitial lung diseases with incredible accuracy. When combined with RWD, we can see how these imaging biomarkers correlate with long-term patient outcomes.
- Precision Medicine and Multi-omics: By analyzing multi-omic data (genomics, proteomics, metabolomics) alongside clinical RWD, we can identify specific patient subgroups that will respond best to a particular therapy. This minimizes the “trial and error” of traditional prescribing and reduces the risk of adverse events.
Causal Inference: Moving Beyond Correlation
One of the biggest challenges in RWD is that it is observational, not experimental. This means correlation does not always equal causation. Advanced analytics now use causal inference techniques, such as propensity score matching and G-computation, to simulate the conditions of a randomized trial. These methods help account for “confounding by indication”—the fact that sicker patients are often given stronger drugs, which can make the drug look less effective than it actually is if not properly adjusted for.
Scaling Patient Analytics with Federated Learning
Scaling these insights across an entire organization requires more than just a smart algorithm. It requires a robust infrastructure that addresses the “Three Pillars of Success”:
- People: You need specialized teams of data scientists, biostatisticians, and epidemiologists who understand both the technology and the clinical context. The “human in the loop” is essential for interpreting AI results.
- Process: Governance is key. We need Standard Operating Procedures (SOPs) for data access, privacy, and ethical AI use to ensure results are reproducible and fair. This includes bias detection to ensure AI models don’t disadvantage specific demographic groups.
- Technology: This is where the “Data Lakehouse” and Federated Learning come in. Modern platforms like Lifebit use a Trusted Research Environment (TRE) to allow researchers to work on data where it lives. In a federated approach, the AI model travels to the data, learns from it locally, and only sends back the “insights” (mathematical weights) to a central server. This solves the privacy problem—you never move sensitive patient records across borders or institutional firewalls.
Research has shown that Big Data in health research is the foundation for these scientific findings, paving the way for a revolution in public health.
Strategic Benefits for Pharmaceutical Manufacturers
For manufacturers, real-world data analytics in healthcare is no longer a “nice-to-have” experimental project. It is a strategic necessity that impacts the bottom line and, more importantly, patient lives. By leveraging RWD, pharma companies can move from a “one-size-fits-all” blockbuster model to a more targeted, value-driven approach.
Optimizing the Product Lifecycle with Real-World Data Analytics in Healthcare
RWD provides value at every single stage of a drug’s life, from early discovery to post-market surveillance:
| Stage | RWD Application | Strategic Benefit |
|---|---|---|
| Late-Stage Pipeline | Identify target markets and comorbidities | Improve forecast accuracy and trial design; reduce recruitment time |
| Pre-Launch | Identify early adopter HCPs and patient clusters | Optimize market entry and competitive positioning; refine messaging |
| Post-Launch | Monitor real-world safety and effectiveness | Rapidly detect adverse reactions; support line extensions for new indications |
| Market Maturity | Demonstrate long-term value to payers | Secure better reimbursement and pricing models; defend market share |
External Control Arms (ECAs) and Synthetic Control Arms
In the late-stage pipeline, RWD can save hundreds of millions of dollars by accelerating trials through the use of External Control Arms (ECAs). In traditional trials, half the patients receive a placebo. In rare diseases or oncology, this can be ethically challenging or difficult for recruitment. By using RWD from previous trials or EHRs to create a “synthetic” control group, researchers can compare the new treatment against a historically matched population. This reduces the number of patients needed for a trial and speeds up the path to regulatory approval.
Demonstrating Value and Improving Outcomes
In an era of value-based care, payers (insurance companies and governments) want proof that a drug works in the real world before they agree to cover it. RWD allows manufacturers to:
- Detect Adverse Reactions Early: Data mining across heterogeneous datasets can spot safety signals months or years before they would appear in traditional reporting. This allows for faster label updates and improved patient safety.
- Improve Adherence and Persistence: By analyzing pharmacy and claims data, we can understand why patients stop taking their medicine—whether due to cost, side effects, or lack of efficacy—and develop targeted interventions to keep them on track.
- Support Rare Disease Approvals: When a disease is so rare that a large Randomized Controlled Trial (RCT) is impossible, RWE from registries can provide the primary evidence for regulatory approval. This has already been seen in several orphan drug approvals where RWE served as the primary evidence for efficacy.
- Value-Based Contracting: Manufacturers can enter into agreements where payment is tied to actual patient outcomes (e.g., a reduction in hospitalizations) as measured by RWD, aligning the interests of the manufacturer, the payer, and the patient.
Navigating Global Data Landscapes and Regulatory Standards
The world of healthcare data is a patchwork of different rules, formats, and sources. Navigating this landscape requires a deep understanding of local markets and global standards. As data becomes more fragmented, the ability to harmonize it becomes a competitive advantage.
The Global RWD Ecosystem: US, UK, and Israel
Different regions offer unique opportunities for real-world data analytics in healthcare:
- United States: The US offers massive claims databases (like Optum or Truven) and rich ambulatory EHR data. The FDA has been a global leader, providing guidance on using EHRs and claims data for regulatory decisions. The 21st Century Cures Act has created a formal pathway for RWE to support new drug indications, particularly in oncology.
- United Kingdom: The UK boasts some of the world’s best longitudinal patient data through the National Health Service (NHS). Resources like IQVIA Medical Research Data (IMRD) and Hospital Episode Statistics (HES) allow us to track patient journeys from primary care to hospital discharge with incredible detail. The UK’s focus on Trusted Research Environments (TREs) has set a global standard for secure data access.
- Israel: Israel’s healthcare system has been digitized since the early 2000s, covering nearly the entire population through four main Health Maintenance Organizations (HMOs). This makes it a “living laboratory” for high-quality research on vaccine effectiveness and chronic disease management, as seen during the COVID-19 pandemic.
- Canada: Canada offers diverse provincial administrative and EMR datasets. Because healthcare is managed at the provincial level, these datasets provide a robust view of public healthcare delivery and long-term outcomes in a single-payer system.
Overcoming Challenges in Data Integration and Sovereignty
Despite the abundance of data, three major problems remain that require technical and strategic solutions:
- Privacy and Data Sovereignty: Regulations like GDPR (Europe) and HIPAA (US) are strict, and many countries now have “data residency” laws that prevent patient data from leaving their borders. We solve this through federated AI, which allows for multi-country research without ever moving the data out of its original secure environment. This respects data sovereignty while enabling global insights.
- Standardization and the OMOP CDM: Without common languages like LOINC for labs or RxNorm for drugs, data cannot be aggregated. Participating in networks like OHDSI (Observational Health Data Sciences and Informatics) and adopting the OMOP Common Data Model is critical. This transforms disparate data into a unified format, allowing a single analytical script to run across dozens of different databases worldwide.
- Interoperability and Tokenization: Data fragmentation is the enemy of insight. We use “tokenization”—creating unique, de-identified alphanumeric strings—to link a patient’s EHR record with their claims data and their wearable logs. This creates a 360-degree view of the patient without ever revealing their identity, maintaining the highest standards of privacy while maximizing the utility of the data.
Frequently Asked Questions about RWD Analytics
What is the difference between RWD and RWE?
Think of RWD as the “raw ingredients” (the records, the logs, the claims) and RWE as the “finished meal.” Real-world data is the information collected during routine clinical practice; real-world evidence is the clinical proof and actionable insight derived from the rigorous analysis of that data. You need high-quality analytics and statistical validation to turn one into the other.
How do regulatory bodies like the FDA use real-world data?
The FDA uses RWE to monitor post-market safety, support the approval of new indications for existing drugs, and fulfill post-approval study requirements. Under the 21st Century Cures Act, they are increasingly looking at how RWE can speed up the approval process for drugs where traditional trials are difficult to conduct, such as in rare diseases or for pediatric populations.
What are the biggest challenges in scaling healthcare analytics?
The “Big Three” are data quality (it’s often messy, incomplete, or biased), privacy (navigating different global laws and data residency requirements), and the “skills gap” (the need for experts who understand both data science and clinical medicine). Using a federated platform like Lifebit helps overcome these by providing a secure, standardized environment for collaboration without moving sensitive data.
How do you handle missing data in real-world datasets?
Missing data is a common challenge in RWD because, unlike clinical trials, data isn’t collected for research purposes. Analysts use techniques like multiple imputation, where statistical models “fill in” missing values based on other available data, or sensitivity analyses to ensure that the missing information doesn’t bias the final results.
What is the role of tokenization in patient privacy?
Tokenization is a process that replaces sensitive patient identifiers (like names or social security numbers) with a unique, encrypted “token.” This allows researchers to link data about the same patient from different sources (e.g., linking a pharmacy record to a hospital record) without ever seeing the patient’s actual identity, ensuring HIPAA and GDPR compliance.
Conclusion: The Future is Federated
We are moving past the era of “Big Data” as a buzzword and into the era of “Actionable Evidence.” The true power of real-world data analytics in healthcare isn’t just in the volume of data we collect, but in our ability to analyze it securely, ethically, and across institutional boundaries. The shift from centralized data silos to decentralized, federated models is the most significant evolution in medical research in decades.
At Lifebit, we believe the future of healthcare research is federated. By using Trusted Research Environments (TREs) and a Trusted Data Lakehouse (TDL), we enable biopharma companies, public health agencies, and governments to collaborate on global biomedical and multi-omic data in real-time. This architecture ensures that data stays under the control of the original provider while still allowing the global research community to extract life-saving insights.
This isn’t just about faster analytics; it’s about building a more patient-centric healthcare system. It’s about a world where every piece of data—from a clinical note to a genomic sequence—contributes to a faster cure, a more accurate diagnosis, and a better outcome for patients everywhere. The technology to bridge the gap between data and evidence is here; the next step is for the industry to embrace these new standards of collaboration.
Ready to see how federated AI can transform your RWD strategy? Let’s talk about building a more secure, scalable future for your research.
News Flash: How Federated AI Cut Data Review Time by 80%
Why wait months for data access? See how Lifebit’s Trusted Research Environment is helping researchers move from variant to target in record time, while keeping 100% of patient data secure. Learn more at Lifebit.ai

