Breaking down barriers: increasing diversity in clinical trials

Introduction
Clinical trials are the cornerstone of medical advancements. They provide the critical data needed to develop new treatments and improve patient outcomes. However, there is a lack of diversity among participants in clinical trials, which negatively impacts the results and impact of trials.
This lack of diversity can skew results and lead to treatments that are less effective for certain populations. Significant barriers exist that hinder the inclusion of diverse populations in clinical trials, ranging from socioeconomic factors to systemic issues within the healthcare industry. This blog explores the challenges and strategies for increasing diversity in clinical trials, including using real world data (RWD).
The imperative for diversity in clinical trials
Without diverse representation in clinical trials, we risk developing treatments that are less effective for certain populations. This can exacerbate health disparities. Moreover, the generalizability of clinical trial results is compromised without diversity. The findings may not apply to the broader population.
By maximizing diversity in clinical trial three key advantages can be gained:
- Firstly, it ensures that research findings are applicable to a broader range of people, including those from various ethnicities, ages, genders, and socioeconomic backgrounds.
- Secondly, certain diseases may disproportionately affect specific demographic groups, and without diverse participation, the effectiveness of treatments in these populations remains uncertain.
- Thirdly, genetic variations among different populations can influence drug metabolism and response, highlighting the importance of inclusivity in research studies.
In essence, diversity in clinical trials is crucial for the development of safe, effective, and equitable healthcare solutions.
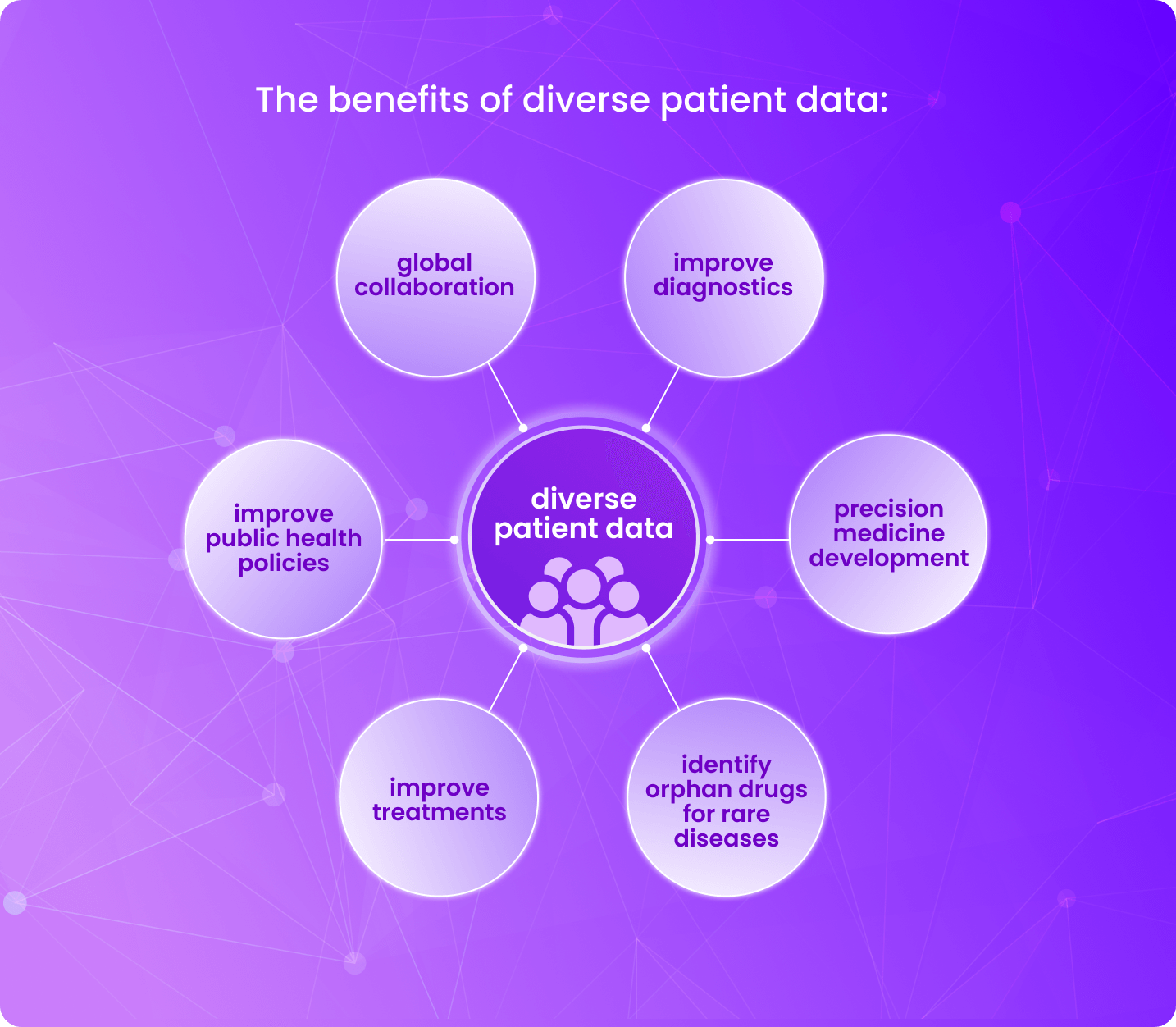
Historical context and current challenges to diverse clinical trials
Historically, clinical trials have been dominated by certain demographic groups, predominantly, white, male, and middle-aged participants. This lack of diversity is a result of various barriers including socioeconomic, cultural, and systemic factors.
For instance, minority populations often have limited access to healthcare. This restricts their ability to participate in clinical trials. Moreover, there is a lack of trust in medical research within certain communities, due to historical injustices and unethical research practices. However, encouraging research on the topic suggests that this is changing for the better.
Barriers to achieving diversity in clinical trials
Achieving diversity in clinical trials is a complex task which involves overcoming numerous barriers.
- Socioeconomic status can significantly influence an individual’s ability to participate in clinical trials. Lower income individuals may lack the resources to take part.
- Cultural factors also play a role. For instance, language barriers can prevent individuals from understanding the trial process. Moreover, cultural beliefs and attitudes towards healthcare can affect participation. This is particularly true in communities with a history of medical mistrust.
- Systemic issues within the healthcare system can also hinder diversity. For example, clinical trials are often conducted in urban, academic medical centers.This can limit access for individuals living in rural areas or those without reliable transportation.
- The design of clinical trials can be a barrier. Trials often have strict eligibility criteria. This can exclude individuals with certain health conditions or those taking specific medications.
Strategies for Enhancing Diversity in Clinical Trials
Establishing Recruitment and Retention Approaches
Recruitment and retention of diverse populations is a key strategy. This involves reaching out to underrepresented communities, including partnering with community organizations to help bridge the gap between researchers and potential participants. Moreover, retention strategies can include regular follow-ups and providing support throughout the trial process. Trust-building is a key aspect of this. Researchers must work to build trust with diverse communities by addressing historical injustices and ensuring transparency in research practices.
Leveraging New Technologies
Technology plays a crucial role in ensuring diversity in clinical trials by facilitating broader participant recruitment through digital platforms and social media, reaching underrepresented populations. Additionally, advanced data analytics and machine learning algorithms help identify and address biases, ensuring more inclusive and representative study populations.
Ensuring Robust Ethical and Security Measures
Ethical considerations in clinical trials involve ensuring informed consent, protecting participant privacy, and maintaining transparency to uphold the dignity and rights of participants. Security considerations focus on safeguarding sensitive data from breaches and ensuring the integrity of trial results through robust data protection measures. By ensuring people from all backgrounds have trust that their data will be protected could help increase the likelihood of participation.
Using Pre-existing, Diverse Real World Data Sets
By using RWD, researchers can gain additional insight into how different patient subgroups respond to medicines in real-world settings. This facilitates our understanding of a drug’s safety and effectiveness across a range of populations.
RWD can assist in improving equality in access to research studies for older persons, people with multiple medical conditions, and members of minority populations—populations that are frequently left out of traditional studies. Robust RWD exploitation can be used to supplement clinical trials and effectively enhance diversity.
Featured Resource: Read our white paper where we discuss pioneering approaches for pharmaceutical companies to effectively leverage RWD in clinical trials and healthcare.
Summary
Streamlining access to fragmented data for researchers could facilitate the identification of new targets, thereby enhancing the likelihood of success. Additionally, identifying suitable cohorts for clinical trials is paramount in advancing research efforts in neurology.
About Lifebit
Lifebit’s federated technology provides secure access to deep, diverse datasets, including oncology data, from over 100 million patients. Researchers worldwide can securely connect and analyze valuable real world, clinical, and genomic data in a compliant manner.

