Clinical Trial Patient Data 101: Your Guide to Medical Research

Clinical Trial Patient Data: Empowering 101 Guide
Why Clinical Trial Patient Data Matters for Medical Progress
Clinical trial patient data represents the foundation of modern medical breakthroughs, from life-saving cancer treatments to groundbreaking therapies for rare diseases. This data, collected from millions of volunteers worldwide, drives scientific innovation and helps researchers develop safer, more effective treatments for patients everywhere.
What is clinical trial patient data?
- Patient information collected during medical research studies
- Health outcomes measured throughout treatment periods
- Safety data tracking side effects and adverse reactions
- Demographic details ensuring diverse representation in research
- Biomarker measurements from blood tests, imaging, and tissue samples
Key uses include:
- Developing new medicines and medical devices
- Improving existing treatments and dosing guidelines
- Understanding how treatments work in different patient populations
- Supporting regulatory approvals for new therapies
- Generating real-world evidence for ongoing safety monitoring
Pfizer’s data transparency initiative calls clinical trial volunteers the “unsung heroes of medicine,” and their contributions are vast. Today, platforms like ClinicalTrials.gov list over 300,000 research studies, and secure networks provide access to data from over 80 million patient lives.
However, sharing this data presents challenges. Organizations must steer complex privacy regulations, ensure proper anonymization, and maintain secure research environments, all while respecting participant rights and advancing scientific findings.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where I’ve spent over 15 years developing secure platforms that enable researchers to access and analyze clinical trial patient data across federated environments. My work focuses on breaking down data silos while maintaining the highest standards of privacy and regulatory compliance.
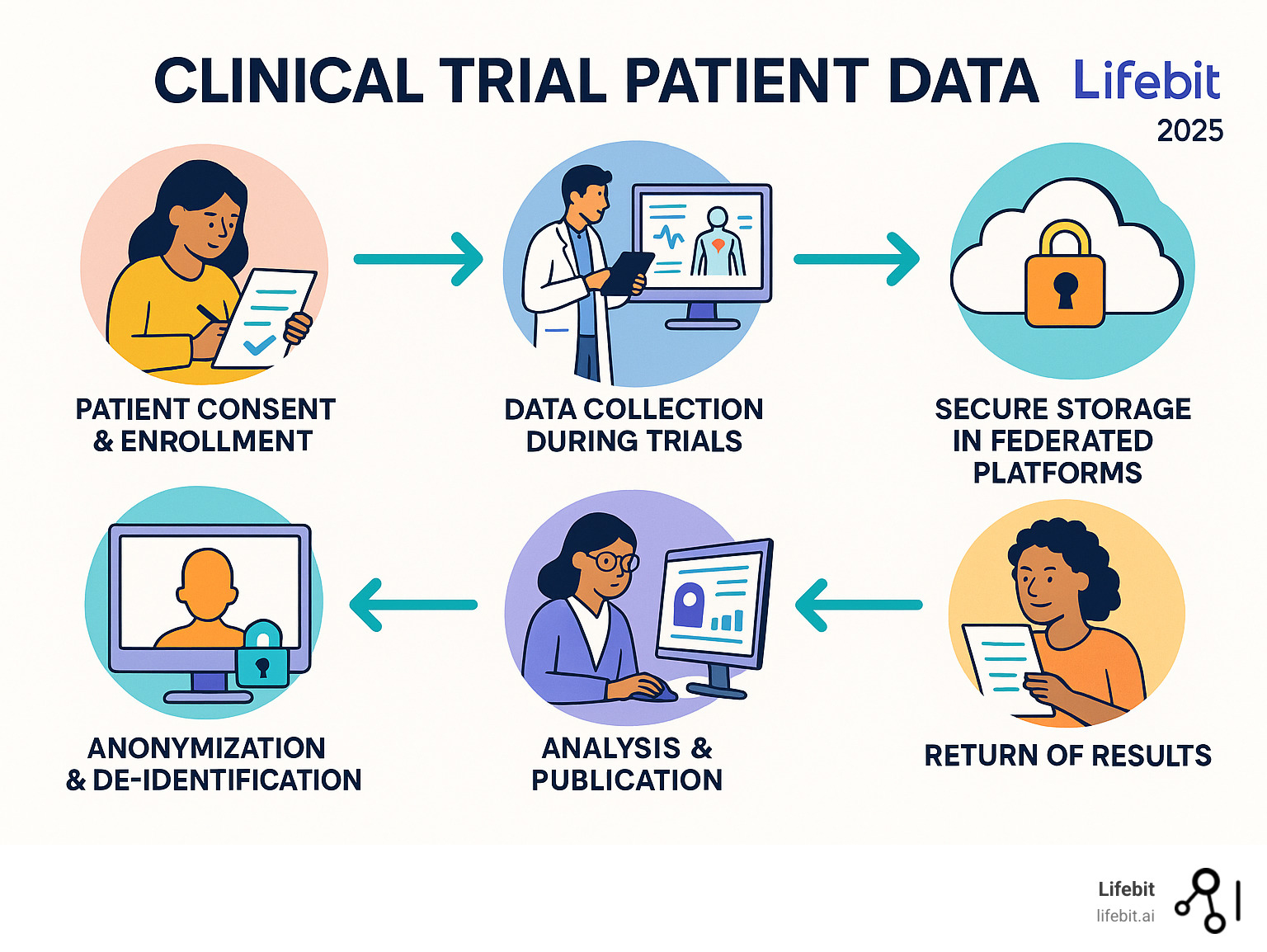
Glossary for clinical trial patient data:
The Importance of Sharing and Returning Clinical Trial Data
Sharing clinical trial patient data is the backbone of modern medical progress. It accelerates innovation by allowing researchers to build on existing findings rather than starting from scratch. This approach also helps avoid duplicate trials, saving resources and preventing patients from being unnecessarily exposed to experimental treatments. Furthermore, when independent researchers re-analyze data and confirm results, it strengthens confidence in the findings, leading to improved medical care for everyone.
There’s also a profound ethical responsibility. The millions who volunteer for trials deserve to see their contributions bear fruit. These individuals share personal health information, sometimes facing risks, to advance medical knowledge. This establishes a partnership between participants and researchers, where transparency is paramount. Industry initiatives, often championed by patient advocacy groups, are now increasingly focused on providing plain-language summaries to make complex findings accessible and honor this partnership.
Why Returning Data to Participants Matters
Returning data to participants is a meaningful way to honor their investment in research. It fosters patient empowerment by providing clear summaries of trial results, giving them insights into their health and how they made a difference. This transparency builds trust in the research community, encourages long-term engagement, and is a tangible way of recognizing that their contribution truly mattered. It transforms the participant from a passive subject into an active partner in the research enterprise, which can lead to higher rates of participation in future studies.
How Data Sharing Fuels Scientific Breakthroughs
The power of data sharing shines in enabling new findies. Secondary analysis allows researchers to ask new questions of existing data, often generating novel hypotheses for future studies. These insights can inform more efficient trial designs and improve drug safety surveillance by identifying rare side effects across pooled datasets. For example, by combining data from dozens of cardiovascular trials, researchers have been able to refine treatment guidelines for blood pressure and cholesterol management, impacting millions of patients globally. This type of meta-analysis would be impossible without a culture of data sharing.
One of the most exciting frontiers is real-world evidence (RWE) generation, where clinical trial patient data is combined with information from sources like electronic health records (EHRs), insurance claims databases, and even data from wearable devices. This provides invaluable insights into how treatments perform in diverse, real-world populations over the long term, outside the controlled environment of a clinical trial. RWE can be used to support regulatory decisions, such as expanding a drug’s approved use, or to monitor long-term safety and effectiveness. This holistic view helps bridge the gap between clinical research and everyday clinical practice.
The impact is remarkable. Data sharing platforms have facilitated access to thousands of trials involving millions of participants, leading to hundreds of new scientific publications. Each shared dataset holds the potential for findies that could change lives.
How Patient Data is Protected and Anonymized
The power of clinical trial patient data comes with the profound responsibility of safeguarding patient privacy. Our approach prioritizes robust security and strict adherence to global privacy regulations, ensuring that the potential of data is open uped without compromising individual rights.

At the core of data protection are sophisticated methods for anonymization and de-identification, governed by stringent legal frameworks.
Advanced Anonymization and De-identification Methods
Simple removal of names and addresses is not enough. Modern techniques provide mathematical guarantees of privacy.
- Anonymization Techniques: These methods permanently remove or encrypt personal identifiers so that the data cannot be linked back to an individual. This includes methods like k-anonymity, which ensures any individual in a dataset cannot be distinguished from at least ‘k-1’ other individuals. Another technique, l-diversity, extends this by ensuring that for any group of indistinguishable individuals, there are at least ‘l’ different values for each sensitive attribute (like a specific diagnosis), preventing inference attacks.
- De-identification Standards: This involves removing or masking direct and indirect identifiers. The HIPAA Privacy Rule provides two paths: the “Expert Determination” method, where a statistician certifies the risk of re-identification is very small, and the “Safe Harbor” method, which involves removing 18 specific identifiers (e.g., names, geographic subdivisions smaller than a state, all elements of dates directly related to an individual).
- Differential Privacy: A cutting-edge approach that adds carefully calibrated statistical noise to a dataset. This allows researchers to perform queries and get accurate aggregate results while making it mathematically impossible to determine whether any single individual’s data was included in the dataset, offering one of the strongest forms of privacy protection.
- Redaction of Personal Information: In clinical study reports and other documents, sensitive personal information is carefully redacted to protect privacy before sharing.
Regulatory Frameworks
Strict laws govern how clinical trial patient data is handled, mandating stringent security and privacy controls.
- General Data Protection Regulation (GDPR): In Europe, GDPR sets a high bar for data protection. While it heavily restricts the processing of personal data, Article 89 provides specific safeguards and derogations for data used for scientific research purposes, allowing for its use under strict conditions like pseudonymization and robust security.
- Health Insurance Portability and Accountability Act (HIPAA): In the United States, HIPAA’s Privacy Rule governs the use and disclosure of Protected Health Information (PHI). It explicitly permits the use of de-identified data for research without patient authorization, as it is no longer considered PHI.
Key Principles for Responsible Data Sharing
Responsible data sharing is built on trust and ethical guidelines. We adhere to principles that emphasize:
- Protecting Patient Privacy: The non-negotiable cornerstone is ensuring individual participants cannot be identified.
- Independent Review Panels: Research proposals are reviewed by independent panels to ensure scientific legitimacy and soundness.
- Data Use Agreements (DUAs): Researchers sign legally binding agreements specifying how data can be used and the required security measures.
- Controlled Access Environments: Data is accessed within highly secure environments, like our Trusted Research Environments (TREs). This ensures data never leaves its secure location and is only accessible to authorized researchers.
- We also follow guidance on data protection from organizations like PHUSE, which has developed model approaches for the protection of personal data in clinical documents.
Challenges and Considerations in Data Sharing
While beneficial, data sharing has challenges we actively work to overcome:
- Data Standardization: Harmonizing data from various systems and formats is essential. This is being addressed through the adoption of common data models like the CDISC (Clinical Data Interchange Standards Consortium) standards for clinical trials and the OMOP (Observational Medical Outcomes Partnership) Common Data Model for observational data.
- Interoperability: Ensuring different platforms can communicate seamlessly is a complex technical challenge that requires standardized APIs and data formats.
- Data Quality: Robust quality checks are necessary to ensure shared data is accurate, complete, and reliable for analysis.
- Balancing Transparency with Privacy: Striking the right balance is an ongoing challenge. The risk of re-identification, sometimes called the “mosaic effect” where combining multiple anonymized datasets can inadvertently reveal an individual’s identity, is a real concern. This is where federated governance models are crucial.
- Federated Governance Models: Our federated approach allows data to remain at its source while enabling secure analysis. This distributed control improves security, maintains data ownership, and directly addresses the privacy-transparency balance by preventing the aggregation of raw data in a central location.
The Ecosystem of Clinical Trial Patient Data
Think of clinical trial patient data as the beating heart of a vast, interconnected network. This ecosystem brings together a diverse group of stakeholders, each with a critical role: patients who generously volunteer their time and health information, researchers hungry for insights, healthcare organizations committed to advancing medicine, regulatory bodies that ensure safety and efficacy, and secure data platforms that make it all possible. The synergy between these players is what transforms individual data points into life-changing medical progress.

What makes this ecosystem truly remarkable is how it’s changing medical research through global collaboration and data transparency initiatives. We’re witnessing a fundamental shift – from isolated studies to connected research that amplifies the impact of every participant’s contribution.
Key Stakeholders in the Data Ecosystem
Understanding the ecosystem requires recognizing the distinct contributions of each participant:
- Patients and Volunteers: The foundation of all clinical research. They provide the essential data and lived experience needed to evaluate new treatments.
- Pharmaceutical and Biotech Companies (Sponsors): They fund, design, and oversee the majority of clinical trials with the goal of developing new, marketable therapies.
- Contract Research Organizations (CROs): Specialists hired by sponsors to manage the complex logistics of a clinical trial, including site selection, patient recruitment, data collection, and monitoring.
- Academic Medical Centers and Research Institutions: These are often the sites where trials are conducted. Their physician-scientists not only run sponsor-led trials but also initiate their own research to answer fundamental scientific questions.
- Regulatory Agencies (e.g., FDA, EMA): Government bodies that set the rules for clinical trials and review the submitted clinical trial patient data to determine if a new drug or device is safe and effective for public use.
- Patient Advocacy Groups: These organizations play an increasingly vital role by representing the patient voice, assisting with trial recruitment, and advocating for policies that promote data sharing, transparency, and patient-centric research.
Major Registries for Finding Clinical Trials
When patients or researchers need to find clinical trials, several key registries serve as their starting points.
- ClinicalTrials.gov is the largest registry, maintained by the U.S. National Library of Medicine, with over 300,000 research studies from more than 200 countries.
- The WHO International Clinical Trials Registry Platform acts as a central portal, providing a single point of access to various national and international registries.
- The EU Clinical Trials Register focuses on European research, listing over 34,000 trials conducted within the EU/EEA.
- The ISRCTN registry accepts all types of clinical research and currently lists over 18,000 studies.
- The Pan African Clinical Trial Registry ensures visibility for clinical research happening in Africa.
Secure Access to Clinical Trial Patient Data
Finding trials is one thing; accessing the actual clinical trial patient data for research requires sophisticated secure environments. These platforms enable responsible research through a structured process. Typically, researchers submit detailed proposals that are reviewed by independent panels to ensure the research is scientifically sound and ethically responsible before access is granted.
Our approach at Lifebit takes this concept even further. Our federated AI platform provides secure, real-time access to global biomedical and multi-omic data without ever moving sensitive information from its original location. Through our Trusted Research Environment and Trusted Data Lakehouse components, researchers can analyze data from thousands of clinical trials while the information stays safely within our secure infrastructure. This means faster insights, AI-driven safety surveillance, and collaboration that spans institutions without compromising security.
Industry Initiatives Improving Data Transparency
The pharmaceutical industry is actively working to improve data transparency, honoring the contributions of trial participants.
Collaborative efforts have developed model approaches for anonymization, with organizations like TransCelerate BioPharma’s Clinical Data Transparency initiative creating practical guidance for responsible data sharing. Other major platforms have emerged, such as Project Data Sphere®, an independent, not-for-profit initiative focused on sharing historical cancer research data, and Vivli, a non-profit that has created a global data-sharing and analytics platform for clinical trials across all diseases. Creating layperson summaries has also become a priority, changing complex jargon into understandable information for participants. This cooperation and commitment to advancing data transparency standards builds trust and encourages more people to participate in research, creating a positive cycle that benefits everyone.
How Patients and Researchers Can Access Data
The world of clinical trial patient data can seem complex, but clear pathways exist for both patients and researchers. For patients, it’s about understanding their rights and finding the right trials. For researchers, it’s about crafting strong proposals and working within secure, ethical frameworks to advance science.
A Guide for Patients and Volunteers
If you’re considering joining a clinical trial, you’re taking an important step toward advancing medicine. The good news is that finding relevant studies and understanding your role has never been easier.
- The Informed Consent Process: Before you can participate in any trial, you must go through a process called informed consent. This is a fundamental ethical requirement. A member of the research team will explain the trial in detail, including its purpose, procedures, duration, potential risks, and potential benefits. You will receive a written document that outlines all of this information. This is your opportunity to ask questions. Your consent must be voluntary, and you have the right to withdraw from the study at any time, for any reason, without penalty. This process ensures you are a willing and informed partner in the research.
- Finding a Trial: ClinicalTrials.gov is your best starting point. This comprehensive database lets you search by your specific condition, like cancer or Alzheimer’s disease, and filter by location and recruitment status. Once you find a promising study, understand the trial information thoroughly. Each listing provides essential details about the study’s purpose, eligibility criteria, and contact information.
- Other Resources: ResearchMatch offers another pathway for volunteers. This NIH-funded platform connects willing participants with researchers who need volunteers for their studies. Patient advocacy groups for your specific condition are also excellent resources.
- Consult Your Doctor: Before moving forward, discussing participation with your healthcare provider is crucial. Your doctor can help you weigh the potential benefits and risks based on your personal health history and may know of local research opportunities.
A Guide for Researchers
Accessing clinical trial patient data for secondary research requires a formal, streamlined, and ethically sound approach.
- Formulate a Clear Research Question: Start with a well-defined hypothesis. A vague request for “all data on a disease” is unlikely to be approved. Your question should be specific, and you must be able to justify why the requested dataset is appropriate to answer it.
- Submit a Detailed Proposal: Access is typically granted by a data access committee or an independent review panel. Your proposal is the key to approval and must include several core components:
- Scientific Rationale: A clear description of the research question, its importance, and the testable hypothesis.
- Statistical Analysis Plan (SAP): A detailed plan outlining the statistical methods you will use to analyze the data.
- Team Expertise: Evidence that your research team possesses the necessary qualifications to conduct the research responsibly.
- Ethical Considerations: A statement on how you will protect patient privacy and confidentiality.
- Publication Plan: A commitment to publish the findings, regardless of the outcome, to contribute to the scientific knowledge base.
- Work Within Secure Environments: Once approved, you will access anonymized datasets within secure, controlled environments. Our Trusted Research Environments (TREs) provide this protected space, with built-in tools for advanced analytics and compliance, ensuring the data itself is never moved or exposed.
- Leverage Innovative Methodologies: The availability of rich datasets is enabling new approaches. For instance, the use of External Control Arms (ECAs) is growing. An ECA uses historical clinical trial data or curated real-world data to serve as the comparator group for a new investigational therapy being tested in a single-arm trial. This can be particularly valuable in rare diseases where recruiting a placebo or standard-of-care arm is difficult or unethical. It can accelerate drug development, but requires sophisticated statistical methods to minimize bias.
- Publish Your Findings: Completing the circle of scientific progress by publishing your findings is a critical responsibility, fulfilling the promise made to the original trial participants.
Frequently Asked Questions about Clinical Trial Patient Data
When it comes to clinical trial patient data, you deserve clear answers about how your data is protected and used. Let’s address the most common concerns.
How is my personal information protected when clinical trial data is shared?
Your privacy is legally protected by strong regulations. Strict laws like GDPR and HIPAA mandate privacy controls. The next layer is anonymization and de-identification, a sophisticated process that removes or masks anything that could identify you. Finally, data access is granted through secure platforms to vetted researchers under strict agreements. Our Trusted Research Environments ensure data never leaves its secure location—researchers can analyze it, but not download or copy it without authorization.
Can I get my own data back after participating in a trial?
Yes, and this is a growing trend. Many organizations now provide participants with plain-language summaries of trial results, explaining what the study found. Some companies go further, offering access to individual data through dedicated portals. This reflects a growing recognition that participants are valued partners in advancing medicine.
Where is the best place to start searching for a clinical trial?
ClinicalTrials.gov is the largest and most comprehensive registry, listing over 300,000 studies worldwide. You can search by condition, location, and other criteria, and each listing provides the details needed to make an informed decision. Your doctor or a patient advocacy group are also excellent resources, as they often know about trials relevant to your specific situation.
Conclusion
The journey of clinical trial patient data shows how individual contributions fuel waves of medical progress. We are in an era of collaborative medicine, marked by a shift towards transparency that benefits both science and patients.
Throughout this guide, we’ve seen how robust systems protect patient data. Anonymization techniques and regulations like GDPR and HIPAA are the cornerstones of this protection. At the same time, secure, federated platforms are enabling researchers to collaborate globally and accelerate findy.
The power of federated platforms is that data can remain safely at its source while being accessible for analysis. This allows a researcher in London to collaborate with colleagues in New York without data ever leaving its secure home.
At Lifebit, we are proud to pioneer this change. Our next-generation federated AI platform provides secure, real-time access to global biomedical data, breaking down barriers while upholding the highest standards of privacy.
Our Trusted Research Environments (TREs) and Trusted Data Lakehouses (TDLs) are bridges connecting the global research community, facilitating secure collaboration across five continents.
The collective power of securely shared clinical trial patient data is opening unprecedented opportunities for medical innovation. We’re excited to build this future together—one where every piece of protected data contributes to a healthier world.
Explore how next-generation platforms enable secure access to global biomedical data.

