Everything You Need to Know About Decentralized Clinical Trials

The Shift to Decentralized Trials: Accelerate Research and Cut Participant Burden
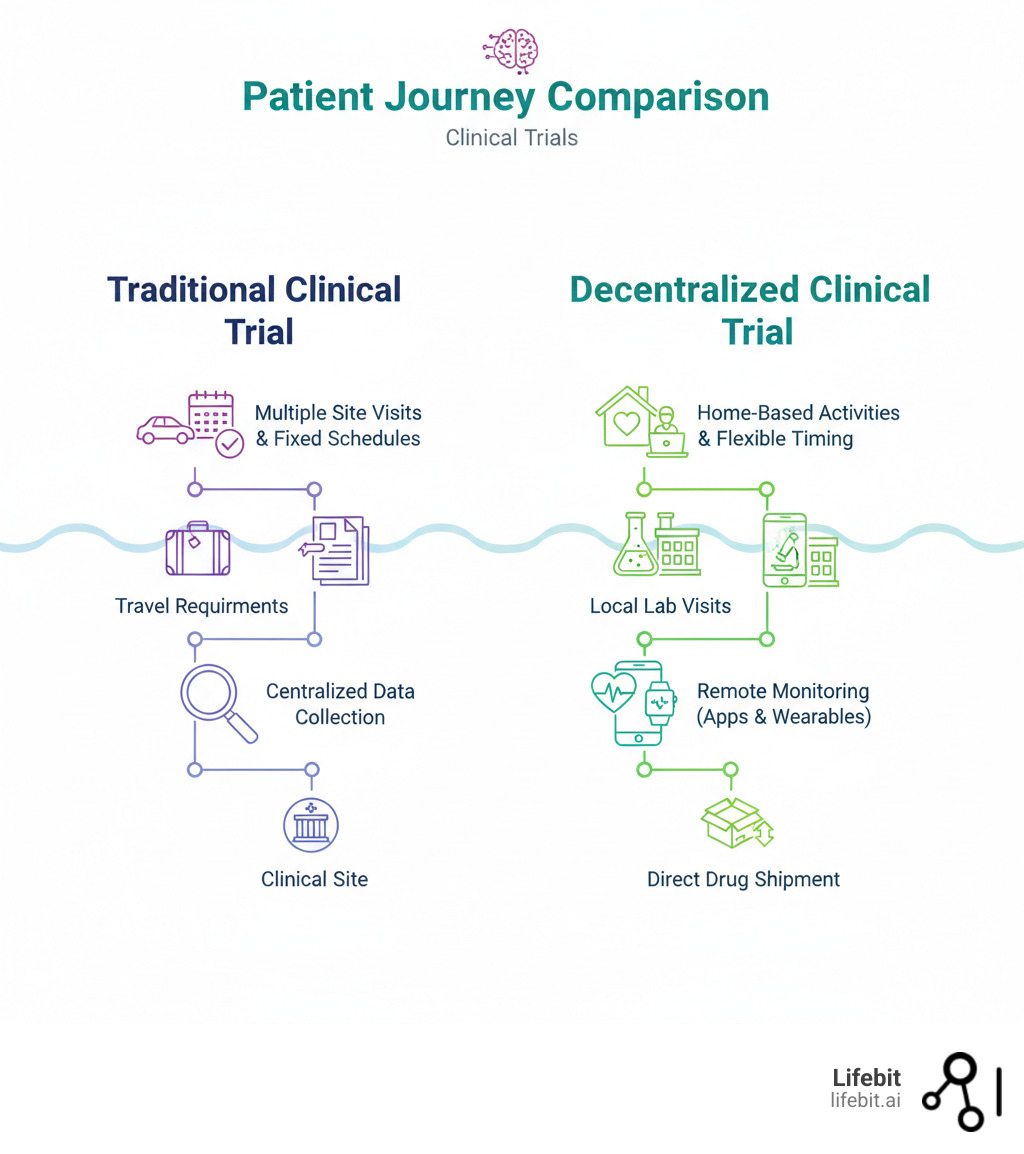
The traditional clinical trial model, built around a handful of centralized academic medical centers, has long been the gold standard for medical research. However, its rigidity has created persistent bottlenecks, with over 80% of trials failing to meet recruitment timelines and nearly a third of participants dropping out before completion. This inefficiency not only delays the arrival of new therapies but also inflates development costs, which can exceed $2 billion per drug.
Decentralized clinical trials (DCTs) represent a fundamental paradigm shift designed to solve these problems. By bringing trial activities directly to the participant, DCTs dismantle the geographic and logistical barriers of the traditional model. According to the FDA, a DCT is any study where some or all trial-related activities occur at a location other than the primary investigator’s physical site. This patient-centric model uses digital health technologies to conduct activities in settings that fit into a participant’s life:
- At home: Using telehealth for consultations, wearables for continuous monitoring, and direct-to-patient shipment for investigational products.
- Locally: Leveraging nearby clinics, pharmacies, or diagnostic labs for procedures like blood draws, imaging, or check-ups.
- Virtually: Employing mobile applications and web-based eConsent platforms for secure data collection and remote permissions.
This flexibility is the engine behind the core benefits of DCTs: faster recruitment, higher retention rates, and access to more diverse and representative patient populations, including rural, elderly, or mobility-impaired individuals. It also enables the collection of continuous, real-world data, providing a much clearer and more accurate picture of a treatment’s safety and effectiveness in a naturalistic setting.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. For over 15 years, I’ve focused on breaking down data silos to accelerate research. The explosion of data from DCTs requires a new kind of infrastructure. Our federated data platforms provide the secure, distributed, and analysis-ready environment that modern DCTs require to succeed at scale.
Why DCTs Are Now Essential: Boost Diversity, Retention, and ROI
For decades, the traditional clinical trial model created immense barriers that limited participation and slowed discovery. The requirement for patients to travel frequently to specific, often distant, sites excluded countless people due to geography, cost, or mobility issues. While the research community recognized the need for a more flexible approach, the COVID-19 pandemic acted as a powerful catalyst, forcing the rapid adoption of remote trial methods and proving that they weren’t just possible—they were often superior.

By moving trial activities closer to participants, DCTs dismantle these long-standing walls. The core philosophy is simple: organize the trial around the participant’s life, not the other way around. This patient-centric approach delivers tangible, quantifiable wins for both participants and researchers, creating a powerful business case for sponsors.
Key Benefits of Decentralization:
-
Greater Accessibility and Enhanced Diversity: Traditional trials have historically struggled with diversity. For example, while Black Americans make up over 13% of the U.S. population, they represent only 5% of trial participants. DCTs directly address this by opening doors for previously excluded populations. With an estimated 70% of Americans living over two hours from the nearest academic trial site, removing the travel burden dramatically expands the potential participant pool. This enables recruitment of more geographically, ethnically, and socioeconomically diverse cohorts, leading to findings that are more generalizable and applicable to the real-world patient population. This isn’t just an ethical imperative; it’s a scientific one, as treatment effects can vary across different demographic groups.
-
Reduced Participant Burden and Improved Retention: Participation in a traditional trial often involves significant hidden costs: time off work, travel expenses, childcare, and more. These burdens are a primary driver of the high dropout rates—up to 30%—that plague many studies. DCTs mitigate this by minimizing or eliminating site visits. For a patient, this could mean the difference between a monthly 4-hour round trip to a hospital and a 15-minute virtual check-in from their living room. This convenience is a critical factor in making participation feasible and sustainable, leading to higher retention rates and ensuring studies maintain their statistical power and validity.
-
Improved Operational Efficiency and ROI: For sponsors, the benefits translate directly to a stronger bottom line. By accessing a wider pool of participants, DCTs can accelerate recruitment timelines by months or even years. Higher retention rates reduce the need to over-enroll patients to compensate for dropouts. Furthermore, decentralization can lower costs associated with managing numerous physical sites, such as site monitoring visits and administrative overhead. This allows for better resource allocation and delivers a clear and compelling return on investment, shortening the path to market for new therapies.
-
Better, Higher-Quality Real-World Data: Wearables and remote monitoring tools capture high-frequency data in a participant’s natural environment, a concept known as ecological validity. Instead of relying on episodic data points collected in the artificial setting of a clinic, researchers can gather a continuous stream of objective information on activity levels, sleep patterns, heart rate variability, and more. This generates novel digital biomarkers that can provide more sensitive and authentic insights into a treatment’s real-world performance and safety profile. As recent scientific research on the value of DCTs highlights, this model offers unprecedented potential for innovation in medical product development.
The DCT Playbook: Models and Tech to Build Your Next Trial
Decentralized clinical trials are not a monolithic, one-size-fits-all solution. They exist on a flexible spectrum, from fully remote studies to hybrid models that blend remote activities with essential in-person visits. The key to a successful DCT is designing a protocol that is tailored to the study’s specific endpoints, the patient population, and the investigational product’s characteristics.
| Feature | Traditional Clinical Trial | Hybrid (“Click and Mortar”) DCT | Fully Remote DCT |
|---|---|---|---|
| Site Visits | All in-person at a central site | Mix of in-person (e.g., for complex procedures) and remote | No in-person site visits required |
| Data Collection | Manual, paper, EDC at site | Mix of remote (wearables, apps) and on-site (imaging, labs) | Predominantly digital, remote sensors, ePROs |
| Patient Interaction | Face-to-face at site | Telehealth, home visits, on-site visits | Telehealth, apps, digital communication platforms |
| IP Management | Dispensed and administered at site | Site-based or direct-to-participant shipment | Direct-to-participant, local pharmacies |
| Participant Reach | Geographically limited to site radius | Regional, national, or global | Potentially global, no geographic limits |
| Examples | Most historical trials | ACTIV-6, many oncology trials | ADAPTABLE study, digital therapeutic trials |
Common DCT Models
Successful trials like the fully remote ADAPTABLE study (which tested optimal aspirin dosage) and the hybrid ACTIV-6 study (which enrolled 26,000 patients across all 50 states to test repurposed drugs for COVID-19) demonstrate the power of these models. You can design your study using several approaches:
- Fully Remote (or Site-less): All activities, from recruitment and consent to data collection and endpoint assessment, are conducted virtually. This model is ideal for low-intervention studies, post-market surveillance, digital therapeutic trials, or studies relying on patient-reported outcomes and data from wearables.
- Hybrid Models: This is the most common and versatile approach, combining the convenience of remote monitoring with essential in-person visits. For example, an oncology trial might require initial in-person visits for complex imaging, biopsies, and the first infusion, followed by remote monitoring of symptoms (ePROs) and telehealth check-ins for the remainder of the study.
- Local Integration: Instead of requiring travel to a central academic site, participants use nearby healthcare infrastructure. This can include local clinics for physical exams, diagnostic labs for blood draws, or infusion centers for treatment administration, all coordinated by the central study team.
- Mobile Support: Trained staff, such as home health nurses or phlebotomists, visit participants at home for specific procedures like sample collection, vital sign measurement, or administering an investigational product.
The Technology Stack Enabling DCTs
This flexibility is powered by a robust, integrated set of Digital Health Technologies (DHTs) that form the trial’s operational backbone.

Key components include:
- Wearable Sensors and Devices: Consumer-grade (e.g., smartwatches) and medical-grade sensors provide continuous, objective data on everything from physical activity and sleep (via accelerometers) to heart rhythm (via ECG) and blood oxygen levels (via SpO2). This creates a rich, longitudinal dataset of a patient’s health status.
- eConsent Platforms: Electronic consent platforms move beyond simple digital signatures. They use interactive elements like videos, quizzes, and layered information to improve participant comprehension of complex trial protocols. They also provide a clear audit trail and ensure the latest version of the consent form is always used.
- Telemedicine Platforms: Secure, HIPAA-compliant video conferencing platforms enable virtual site visits, allowing investigators to conduct consultations, assess certain conditions, and build rapport with participants remotely. This is crucial for maintaining clinical oversight and patient engagement.
- eCOA/ePRO Tools: Electronic Clinical Outcome Assessment (eCOA) and electronic Patient-Reported Outcome (ePRO) tools allow participants to report symptoms, quality of life, and treatment adherence directly via smartphone apps or web portals. This method reduces recall bias and provides real-time data to the study team.
- Integrated Data Systems: The ultimate challenge is to securely capture, harmonize, and analyze data from all these disparate sources—EHRs, wearables, labs, and ePRO apps. At Lifebit, our federated platform is designed specifically for this. It can integrate these diverse data streams in a secure, research-ready environment, applying quality checks and standardization rules while ensuring compliance with global privacy regulations. For more, see the FDA guide to digital health technologies in trials.
DCT Governance & Compliance: A Guide to FDA/EMA Rules and Data Security
While decentralized clinical trials change the location of research, they do not change the fundamental rules of conduct. The core principles of patient safety, data integrity, and ethical oversight remain paramount. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have made it clear that while activities can be decentralized, accountability cannot.

- Sponsor and Investigator Roles: Responsibilities are fundamentally unchanged. Sponsors are still required to ensure proper trial monitoring and management. Investigators retain ultimate responsibility for the trial’s conduct, the protection of participants’ rights and welfare, and the integrity of the data. This oversight must extend to any trial-related activities delegated to local healthcare providers (HCPs), home health staff, or other third-party vendors. Clear contracts, training protocols, and communication plans are essential to ensure all parties adhere to the study protocol and GCP standards.
- IRB/IEC Oversight: All third-party collaborators involved in conducting research activities (e.g., a local clinic performing blood draws) require Institutional Review Board (IRB) or Independent Ethics Committee (IEC) oversight. This ensures that every component of the trial, regardless of where it is performed, adheres to the highest ethical standards for human subject research.
The Regulatory Framework
Both the FDA and EMA have issued comprehensive guidance to support the adoption of DCTs, emphasizing that existing regulatory requirements still apply. The official FDA Guidance on Decentralized Clinical Trials, finalized in 2023, confirms that the standards for ensuring data integrity and protecting human subjects are the same regardless of the trial model. Key takeaways from global regulators include:
- Centralized Accountability: The principal investigator (PI) must maintain oversight of all trial activities, even those performed remotely by other individuals or technologies.
- Technology Validation: Any digital health technology (DHT) used to collect data for primary or secondary endpoints must be ‘fit-for-purpose.’ This means sponsors must verify its accuracy, reliability, and security.
- Inspection Readiness: A physical location (such as the sponsor’s or PI’s office) must be designated and available for regulatory inspections and audits.
Data Privacy and Security: A Critical Pillar
Protecting sensitive participant data as it flows between multiple digital platforms, devices, and locations is a critical challenge. A robust governance framework is non-negotiable.
- eConsent Compliance: Electronic informed consent processes must meet all regulatory requirements (such as FDA’s 21 CFR Part 11) for electronic signatures and records. The process must ensure participants fully understand the study’s risks, benefits, and data handling procedures before providing consent remotely.
- Data Security and Validation: The software, apps, and platforms used must be validated for reliability, security, and functionality. They must comply with stringent data protection regulations like the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in Europe. Sponsors must be transparent with participants about how their data is collected, stored, used, and protected.
This is where Lifebit’s federated technology provides a definitive advantage. Our Trusted Research Environment (TRE) is engineered for secure, real-time access to global biomedical data while upholding the highest standards of data protection and governance. By enabling analysis to happen where the data resides, our platform minimizes data movement, inherently reducing security risks and simplifying compliance with regulations like GDPR, which impose strict rules on cross-border data transfers. We ensure data remains secure and private, even when analyzed across different countries and regulatory jurisdictions.
Solve Top DCT Challenges: Data Integrity, IP Logistics, and Digital Divides
While decentralized clinical trials offer immense benefits, they also introduce unique operational complexities. A successful transition from a traditional to a decentralized model requires proactive planning and the right technological infrastructure to address these new challenges head-on.

Key Challenges and Strategic Solutions:
-
Ensuring Data Quality and Integrity: With data flowing from a multitude of sources—wearables, patient-facing apps, local labs, and electronic health records—ensuring its accuracy, completeness, and reliability is paramount.
- Solution: Implement a centralized, risk-based monitoring (RBM) strategy. Instead of 100% source data verification, RBM uses technology to oversee data streams in near real-time. Automated checks can flag anomalies, missing data, or inconsistencies, allowing a central monitoring team to focus on the highest-risk areas. This maintains the scientific validity required for regulatory acceptance. Lifebit’s platform supports this with built-in data harmonization tools and quality assurance dashboards that provide a unified view of data integrity across all distributed sources.
-
Bridging the Digital Divide: Relying solely on advanced digital tools can inadvertently exclude the very populations DCTs aim to reach—such as the elderly, those in low-income communities, or individuals with low digital literacy.
- Solution: Adopt a multi-faceted approach to participant support. This includes designing apps with simple, intuitive interfaces (UI/UX), providing clear training materials (videos, guides), and offering a tiered technical support system (e.g., chatbot, helpdesk hotline, in-person assistance). For some studies, providing participants with pre-configured devices (like tablets or smartphones) with the necessary apps already installed can be a highly effective strategy. The goal is to make technology an enabler, not a barrier.
Investigational Product (IP) Management and Logistics
Shipping investigational products directly to participants introduces logistical hurdles that require meticulous planning and oversight to ensure patient safety and product integrity.
- Product Suitability: Direct-to-participant (DTP) shipment is best suited for products that are self-administered, stable at ambient temperatures, and have a well-understood safety profile (e.g., oral solids, pre-filled syringes). Biologics or cell therapies requiring complex preparation or strict cold-chain logistics may still necessitate site-based administration.
- Logistics and Stability: Sponsors must partner with specialized couriers to manage the supply chain. This includes using validated packaging that maintains product integrity during transit (e.g., temperature-controlled shippers) and providing participants with clear, simple instructions for receipt, storage, and handling. Technology can be used to track shipments and confirm delivery.
- Investigator Supervision and Safety Monitoring: The principal investigator remains responsible for overseeing IP administration and accountability, even when it occurs remotely. This can be achieved through video calls to observe self-administration, electronic diaries for participants to log doses, and robust systems for real-time adverse event (AE) reporting. A clear plan must be in place to quickly manage and escalate any AEs.
- Medical Devices: For trials involving medical devices, home-use suitability depends on the device’s complexity, risk profile, and the need for user training. Simple, self-administered diagnostic devices may be appropriate for a DCT, while complex therapeutic devices may still require administration by qualified personnel.
Designing for Diversity and Accessibility
DCTs provide a powerful toolkit to create more representative and equitable trials, but this outcome is not automatic. It requires intentional design.
- Overcome Systemic Barriers: Go beyond simply removing travel. Actively recruit in underserved communities by partnering with community health centers, local physicians, and patient advocacy groups. Design the trial to reduce hidden financial burdens by, for example, compensating participants for their time or data costs.
- Bridge the Digital and Cultural Divide: Offer technology, training, and flexible communication options (phone, video, text) to ensure no one is excluded due to a lack of digital proficiency. Provide all participant-facing materials and support in multiple languages and ensure they are culturally competent.
- Build Trust Through Partnership: Trust is the currency of clinical research. Build it by engaging community leaders and local healthcare providers from the outset. When research is embedded within existing, trusted healthcare ecosystems, participants are more likely to feel comfortable and remain engaged. By tackling these challenges proactively, DCTs can deliver on their promise of more inclusive, equitable, and ultimately more powerful research. For more strategies, see this research on improving diversity in clinical trials.
The Future of Research is Federated: AI, RWE, and What’s Next for DCTs
The rapid adoption of decentralized clinical trials is not an endpoint but a catalyst, paving the way for a smarter, more integrated, and data-rich future for medical research. Several key trends are converging to accelerate this transformation, moving the industry toward a model of continuous evidence generation.
-
AI and Machine Learning (AI/ML): DCTs generate massive, continuous data streams that are often too complex for traditional analysis. AI/ML is becoming essential for unlocking their value. Algorithms can be used to optimize trial design, identify ideal participants from real-world data sources, predict individuals at high risk of dropping out, and uncover novel digital biomarkers from sensor data. At Lifebit, we’ve built advanced, privacy-preserving AI/ML analytics directly into our federated platform, allowing researchers to apply these powerful techniques to distributed data without centralizing it, turning raw data into actionable intelligence.
-
Real-World Evidence (RWE): DCTs are powerful engines for generating Real-World Data (RWD)—data on how treatments perform in a patient’s daily life. When analyzed, this RWD produces Real-World Evidence (RWE), which is critical for understanding a product’s true value, safety, and effectiveness outside the controlled confines of a traditional trial. Regulators and payers are increasingly using RWE to support post-market surveillance, label expansions, and value-based reimbursement decisions. DCTs bridge the gap between clinical development and real-world performance.
-
Integrated and Interoperable Platforms: The current landscape of DCT technology is fragmented. The future lies in standardized, interoperable systems where data flows seamlessly and securely between wearables, EHRs, lab systems, and analytics tools. This requires adherence to data standards like FHIR (Fast Healthcare Interoperability Resources). These integrated “trial-in-a-box” solutions will dramatically reduce the complexity and time required to launch and manage a DCT, making them accessible to a broader range of research organizations.
-
Blockchain Technology: While still an emerging technology in this space, blockchain offers a novel approach to enhancing data integrity, transparency, and patient empowerment. Its immutable ledger could provide a verifiable audit trail for everything from patient consent to data access, potentially increasing trial trustworthiness. It also opens possibilities for new models of data ownership, where participants can control and even be compensated for the use of their data.
-
Pragmatic and Hybrid Trials: The future is not a binary choice between “traditional” and “decentralized.” DCTs are ideal for pragmatic trials, which are designed to answer practical questions that matter to real-world patients and doctors, generating evidence that directly informs clinical care. We will see a continued rise in hybrid designs that blend the best of both worlds, using a decentralized approach for convenience while retaining in-person visits for activities that truly require them.
At Lifebit, we are building the federated infrastructure for this interconnected future. Our platform provides secure, real-time access to global biomedical data, enabling large-scale research with built-in data harmonization, AI analytics, and federated governance. Our Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) deliver the secure, collaborative capabilities needed to run complex decentralized and pragmatic studies at scale, ensuring that data security and participant privacy are always the top priority.
Your Next Step in Patient-Centric Research
The rise of decentralized clinical trials marks a definitive turning point for medical research. By putting patients first, we are not just making trials more convenient; we are making them more diverse, more efficient, and more reflective of the real world.
This patient-centric model removes the geographical and financial barriers that have historically limited participation, leading to better science and faster findies. However, the promise of DCTs can only be realized with technology that is secure, scalable, and built for collaboration.
At Lifebit, we provide the federated AI infrastructure that modern research demands. Our platform enables the secure analysis of sensitive biomedical data across distributed environments, ensuring compliance and privacy are never compromised. With our Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), we empower researchers to open up insights from global data without moving it.
As we move toward a future of more inclusive and effective medicine, one thing is clear: patient-centricity is the new standard.
Discover how a federated biomedical data platform can power your next trial

