Decentralized Clinical Trials for CNS Disorders—A Game Changer?

Decentralized Clinical Trials for CNS: 2025 Game Changer
The Growing Need for Innovation in CNS Clinical Research
Decentralized clinical trials for CNS disorders are changing neurological and psychiatric research. The traditional site-based model struggles to serve the more than 1 billion patients worldwide affected by central nervous system disorders, creating a need for more innovative, patient-centric approaches.
Key benefits of decentralized clinical trials for CNS include:
- Reduced patient burden – Eliminates travel requirements and lengthy site visits
- Improved recruitment – Reaches geographically dispersed and underserved populations
- Improved data quality – Captures real-world symptoms and behaviors in natural settings
- Better retention rates – Offers flexible participation options that fit patients’ lives
- Increased privacy – Reduces stigma associated with mental health and neurological conditions
- Cost efficiency – Lowers operational costs while expanding reach
The COVID-19 pandemic accelerated the adoption of remote trial elements, with 76% of pharmaceutical companies implementing decentralized techniques. More than 4,000 CNS clinical trials are expected by 2026, making this the most researched therapeutic area in decentralized settings at 26% of all DCTs.
Traditional CNS trials face challenges like complex patient populations and significant caregiver burden. Patients often struggle with mobility issues, cognitive impairment, or the stigma associated with their conditions—barriers that decentralized approaches effectively address through in-home nursing, telemedicine, and digital monitoring.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology and federated data analysis, I’ve seen how decentralized clinical trials for CNS can open up better patient outcomes through innovative, secure data strategies.
Know your decentralized clinical trials for cns terms:
The Shift to Patient-Centricity: Understanding Decentralized Clinical Trials (DCTs)
For a 68-year-old with Parkinson’s disease, a traditional clinical trial can be an impossible hurdle. It means long, exhausting drives to a distant academic medical center, entire days consumed by appointments, and the logistical challenge of coordinating with a caregiver. For many, the burden is simply too high. Decentralized clinical trials for CNS flip this outdated model on its head. Instead of forcing the patient to conform to the rigid structure of the research site, this approach brings the research directly to the patient, making participation a feasible and empowering reality for those with mobility limitations, cognitive challenges, or geographic constraints.
The traditional site-centric model, which demands physical visits for nearly every trial-related activity, has long created significant barriers for CNS patients. The COVID-19 pandemic served as a powerful catalyst, forcing the industry to adopt remote solutions out of necessity. This rapid, real-world implementation demonstrated the viability of decentralized methods and accelerated the development of supportive regulatory frameworks. The FDA guidance on conducting clinical trials with decentralized elements now formally supports this new paradigm, where trial activities are thoughtfully relocated away from traditional sites and into the patient’s daily life. Instead of asking patients to come to the clinic, DCTs bring the clinic to the patient.
This represents a fundamental philosophical shift from study-centricity to patient-centricity. It’s not merely about convenience; it’s about designing studies around the lived experience of patients. By leveraging a sophisticated ecosystem of digital health technologies, DCTs create flexible, accessible, and more humane research experiences that can finally reach the diverse and underserved populations who have been historically excluded from clinical research.
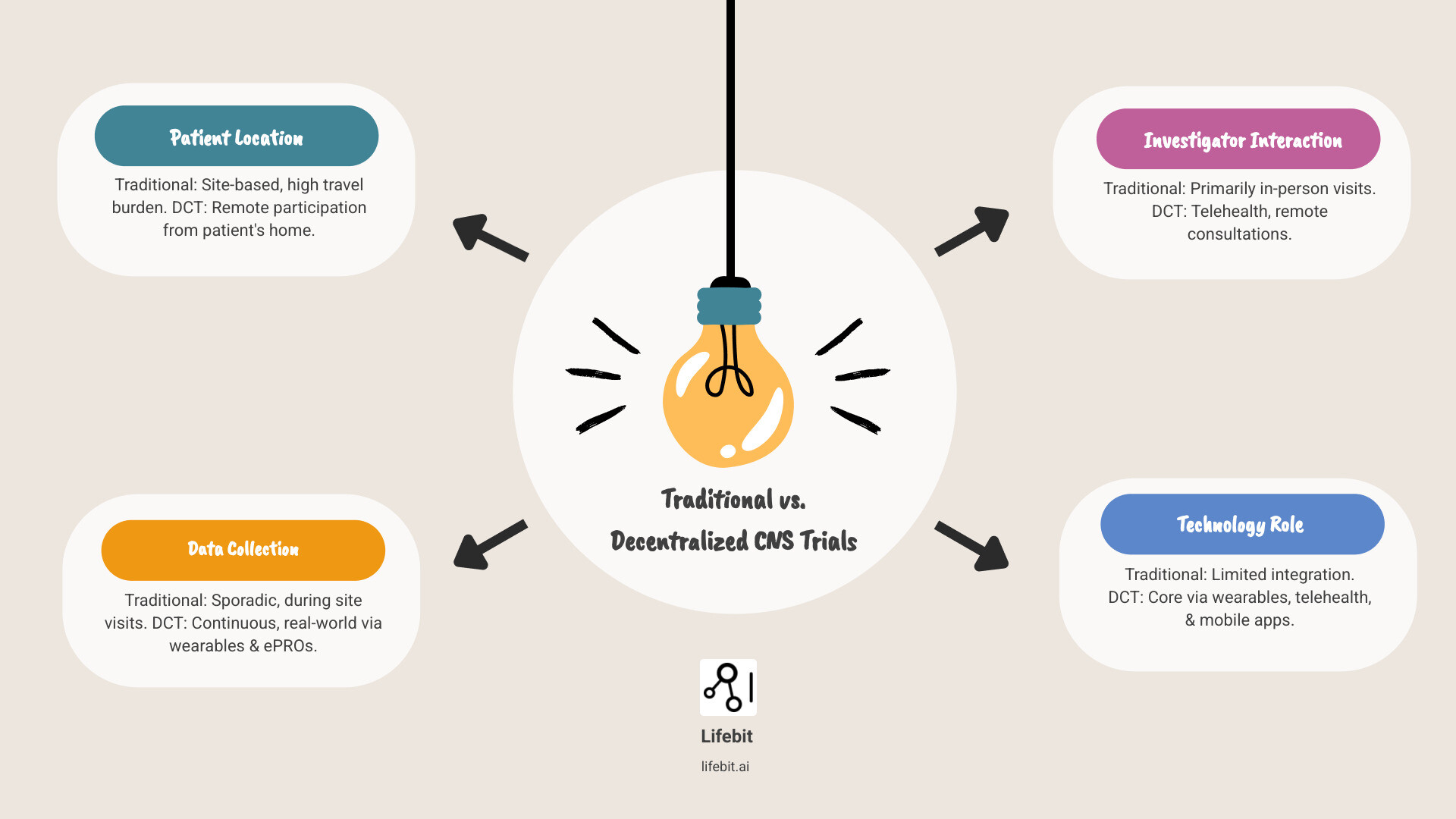
How DCTs Differ from Traditional, Site-Based Trials
The core difference is philosophical. Traditional trials are site-centric, built around the needs and schedules of the research institution, with rigid protocols and in-person data collection. Decentralized clinical trials for CNS are patient-centric, using a toolkit of virtual consultations, wearable sensors, and mobile applications to gather rich, real-world data remotely. Hybrid trials represent the most common and practical application, blending the best of both worlds. They combine the convenience of remote monitoring for routine data collection with essential in-person procedures like complex neuroimaging or initial diagnostic evaluations. The key distinction across this spectrum is flexibility—DCTs adapt to patients’ lives, rather than forcing patients’ lives to adapt to the trial.
| Feature | Traditional Clinical Trials | Decentralized Clinical Trials (DCTs) | Hybrid Clinical Trials |
|---|---|---|---|
| Patient Location | Primarily at designated clinical sites | Primarily at patient’s home or local facilities | Mix of site visits and remote participation |
| Data Collection | In-person, manual data entry, paper CRFs | Remote through digital tools (wearables, ePROs, apps) | Combination of in-person and remote digital data collection |
| Investigator Interaction | Face-to-face at site | Virtual (telehealth, video calls, chat) | Mix of face-to-face and virtual interactions |
| Flexibility | Low, rigid schedules | High, adaptable to patient’s routine | Moderate to High, balances convenience with necessary site visits |
| Technology Use | Minimal, primarily for EDC | Extensive (telemedicine, wearables, ePRO, eConsent, apps) | Moderate to Extensive |
| Patient Burden | High (travel, time off, logistics) | Low (reduced travel, increased convenience) | Moderate (reduced burden compared to traditional) |
| Recruitment Reach | Limited by geography | Broad, inclusive of diverse and remote populations | Broader than traditional, narrower than fully decentralized |
The Technology Backbone: Enabling Remote CNS Research
A sophisticated and integrated technology infrastructure is the backbone of modern remote research, creating a seamless connection between patients, investigators, and the data they generate. Key components include:
- Telehealth platforms: These enable secure, virtual consultations, allowing for remote neurological exams, psychiatric evaluations, and therapeutic sessions like cognitive behavioral therapy (CBT). This dramatically reduces the travel stress and logistical burden on patients and their caregivers.
- Wearable sensors: Medical-grade wearables can continuously and objectively monitor symptoms that are difficult to assess during brief clinic visits. For instance, accelerometers can quantify tremors and dyskinesia in Parkinson’s patients, while actigraphy devices can track sleep patterns and circadian rhythm disruptions common in depression and Alzheimer’s disease.
- Electronic Patient-Reported Outcomes (ePROs): Delivered via smartphone apps, ePROs allow patients to report symptoms, mood, and quality of life in real-time. This high-frequency data capture minimizes recall bias, providing a more accurate picture than asking a patient to remember how they felt weeks ago. In CNS trials, this can involve standardized scales like the PHQ-9 for depression or portions of the UPDRS for Parkinson’s.
- eConsent platforms: Electronic consent tools allow patients to review trial information from the comfort of their home, at their own pace, and with the involvement of family or caregivers. Interactive elements like videos and quizzes can improve comprehension, ensuring a more informed consent process.
- Mobile health apps: These apps often serve as the central hub for the patient, acting as a digital coordinator. They can deliver medication reminders, schedule telehealth visits, provide access to ePRO surveys, and offer a secure channel for communication with the study team.
- Secure data platforms: The vast amount of sensitive health data generated in a DCT requires an exceptionally secure platform. These systems must ensure compliance with regulations like GDPR and HIPAA, and increasingly leverage advanced concepts like federated data analysis to allow for insights to be generated without moving raw data, thus maximizing security and privacy.
When thoughtfully integrated, this technological ecosystem creates a comprehensive, longitudinal, and real-world picture of a patient’s health journey, all without them needing to leave their home.
Why DCTs are Uniquely Suited for CNS Research
Central nervous system (CNS) disorders present a unique and formidable set of challenges for traditional clinical trials, frequently leading to crippling delays caused by recruitment and retention struggles. It is precisely for this reason that decentralized clinical trials for CNS have become the most researched therapeutic area for DCTs, accounting for a remarkable 26% of all such studies. The patient-centric model’s core benefits align perfectly with the specific needs of CNS patients, who often grapple with debilitating mobility issues, progressive cognitive challenges, fluctuating symptoms, and significant social stigma. As a comprehensive analysis of CNS trial challenges confirms, recruitment and retention are the primary bottlenecks, and DCTs are uniquely positioned to dismantle these long-standing barriers.
Overcoming Recruitment and Retention Problems in CNS Trials
Recruiting and retaining participants in CNS trials is notoriously difficult. The burden of participation often outweighs the potential benefits for patients and their families. DCTs directly address these core issues by fundamentally redesigning the participant experience.
- Eliminating Geographic Barriers: By allowing patients to participate from home, DCTs remove the need for difficult, costly, or sometimes impossible travel. This is a game-changer for patients in rural areas, those with limited access to transportation, and individuals whose conditions, like Multiple Sclerosis or advanced Parkinson’s, make travel physically taxing.
- Reducing Caregiver Burden: CNS conditions often necessitate a heavy reliance on caregivers for transportation, scheduling, and support during long site visits. Remote participation significantly lessens this stress on family members, reducing burnout and making long-term trial commitment more sustainable for the entire family unit.
- Improving Access for Diverse Populations: Logistical and financial problems disproportionately affect underrepresented minority and lower-income populations. By removing these barriers, DCTs can help enroll a more representative patient population, improving the generalizability and equity of research findings.
Condition-Specific Advantages:
- Alzheimer’s Disease: Patients often experience disorientation and anxiety in unfamiliar environments. DCTs allow assessments to occur in the comfort of a familiar home setting. Furthermore, the immense burden on caregivers, a primary reason for trial dropout, is substantially reduced.
- Multiple Sclerosis (MS): The unpredictable nature of MS, with its relapsing-remitting symptoms and fatigue, makes fixed appointment schedules challenging. DCTs offer the flexibility for patients to complete assessments when they feel well enough, from their own home.
- Major Depressive Disorder (MDD): Anhedonia, lack of motivation, and social anxiety are hallmark symptoms of depression that create powerful barriers to leaving the house and engaging in clinical research. The privacy and low-effort nature of remote participation can significantly increase enrollment and adherence in this population.
Enhancing Data Quality and Capturing Real-World Evidence
Beyond logistics, DCTs can significantly improve the quality and depth of data collected in CNS research. Traditional trials rely on episodic snapshots of a patient’s condition during brief site visits, which may not be representative of their typical state, especially for conditions with fluctuating symptoms.
- Continuous, Objective Data Collection: Wearables and passive sensors can monitor symptoms 24/7, providing a rich, longitudinal dataset. This allows for a complete picture of disease progression and treatment effect, capturing nuances that are invisible during a 30-minute clinic visit.
- Reduced Recall Bias: By using ePROs for in-the-moment symptom reporting, DCTs yield far more accurate data than retrospective reporting, which is notoriously unreliable, especially in patients with cognitive impairment.
- Greater Ecological Validity: Collecting data in a patient’s natural environment (their home and community) ensures that the findings are relevant to their actual, real-world experience. An improvement on a cognitive test in a quiet lab may not translate to improved function in a busy home, and DCTs help capture this real-world impact.
The Rise of Emerging Digital Biomarkers:
Objective data captured via smartphones and wearables are giving rise to a new class of powerful digital biomarkers that can detect subtle changes in a patient’s condition:
- Gait and Motor Function: Smartphone accelerometers can analyze gait speed, stride length, and balance to track progression in Parkinson’s disease or MS.
- Cognitive Function: Analysis of keyboard interaction patterns (typing speed, error rates, use of backspace) can serve as a sensitive, passive proxy for cognitive processing speed and executive function.
- Vocal and Speech Patterns: Analysis of pitch, tone, and speech rhythm from brief, recorded audio clips can provide objective markers for mood changes in depression or apathy in neurodegenerative disorders.
Reducing Stigma and Improving Patient Privacy
Many neurological and mental health conditions carry a heavy social stigma that can deter individuals from being seen at a specialty clinic or participating in a trial. Decentralized clinical trials for CNS mitigate this concern by allowing participation from the privacy and comfort of one’s own home. Remote consultations and secure digital data submission protect patient anonymity, which can foster more honest and complete reporting on sensitive topics like mood, suicidal ideation, or cognitive decline. When patients feel safe and unjudged, the data they provide is ultimately more reliable and valuable.

Implementing Successful Decentralized Clinical Trials for CNS

Successfully implementing decentralized clinical trials for CNS requires far more than simply deploying technology; it demands a deeply considered, patient-centric strategy from the outset. The most effective trials are not fully decentralized or fully site-based, but rather a thoughtful hybrid. They employ a custom toolkit of remote and in-person elements, carefully selecting the right solution to meet the specific needs of the study protocol, the therapeutic area, and, most importantly, the patient population.
Key Strategies and Technologies in Decentralized Clinical Trials for CNS
Effective DCT strategies for CNS research focus on bringing the trial to the patient in a way that maintains the highest standards of data quality, regulatory compliance, and patient safety. Key operational approaches include:
- In-home nursing visits: For patients with significant mobility issues (e.g., ALS, advanced Alzheimer’s) or for procedures requiring clinical skill, mobile nurses are essential. These professionals can conduct blood draws, administer investigational products, perform physical assessments, and assist patients with technology, all within the patient’s home.
- Direct-to-patient (DtP) drug delivery: Shipping medication directly to a patient’s home eliminates a major source of burden and is a critical factor in improving retention. This requires sophisticated logistics to manage temperature controls (cold chain), track shipments, and confirm receipt by the patient.
- Wearable devices and sensors: To be effective, sponsors must select validated, medical-grade devices that capture data relevant to the trial’s endpoints. The deployment strategy must also include providing patients with pre-configured devices and clear, simple instructions to ensure high adherence and data quality.
- Electronic Patient-Reported Outcomes (ePROs): Implementation goes beyond the technology to include the design of the reporting schedule. A high-frequency schedule (e.g., daily mood ratings) can capture symptom variability, but sponsors must be careful not to create excessive patient burden. Push notifications and user-friendly interfaces are key.
- Digital cognitive assessments: Validated, tablet-based versions of classic neurological tests (e.g., Digit Symbol Substitution Test, Trail Making Test) allow for remote tracking of cognitive function. This reduces the need for frequent, burdensome site visits for neuropsychological testing.
- Gamification and Engagement: To maintain long-term adherence, especially in trials involving younger populations (e.g., ADHD) or those targeting cognitive function, gamified elements can be incorporated into digital assessments and tasks. This can improve engagement and the quality of data collected over time.
The Hybrid Model: Finding the Right Balance for CNS Studies
Fully remote trials are often not feasible or desirable for complex CNS research. Many studies require procedures like high-resolution MRI or PET scans, lumbar punctures for cerebrospinal fluid collection, or complex drug infusions that can only be performed at a specialized site. The hybrid model offers the ideal pragmatic solution, combining the efficiency and convenience of remote monitoring with the scientific necessity of in-person visits.
Consider a hypothetical hybrid trial for an early-stage Alzheimer’s drug:
- Visit 1 (On-site): The patient undergoes a baseline visit at a clinical site for initial diagnosis confirmation, a PET scan to detect amyloid plaques, a lumbar puncture, and a detailed in-person neurological exam. They are also trained on the trial’s digital tools.
- Months 1-11 (Remote): The patient participates from home. They take a daily cognitive test on a provided tablet, wear a sensor to track sleep and activity, and complete a weekly ePRO survey about mood and daily function. A mobile nurse visits quarterly for a blood draw. The investigational drug is shipped directly to their home each month.
- Visit 2 (On-site): At 12 months, the patient returns to the site for a follow-up PET scan and neurological exam to assess the primary endpoints.
This hybrid approach dramatically reduces the patient and caregiver burden from twelve site visits to just two, while still capturing robust, high-frequency data and performing essential on-site procedures.
Centralized vs. Decentralized Rating: A Critical Decision for Sponsors
A critical strategic decision for sponsors in decentralized clinical trials for CNS is how to handle clinical outcome assessments (COAs) that rely on trained raters, such as the ADAS-Cog for Alzheimer’s or the HAM-D for depression.
- Centralized rating involves a small, dedicated team of expert raters who conduct all assessments for the trial, typically via video conference. This model significantly reduces inter-rater variability, leading to cleaner data and increased statistical power. However, it can present logistical challenges with scheduling across time zones and may feel less personal for the patient.
- Decentralized rating relies on trained staff at local sites or designated local healthcare providers to perform the assessments. This can foster a stronger patient-rater relationship and may be more convenient. The major risk is introducing significant data variability, even with extensive training, as different raters may score inconsistently.
Often, a mixed approach is best. A sponsor might use centralized rating for the primary efficacy endpoint where precision is paramount, while allowing for decentralized rating for secondary or safety assessments. Technology can also help bridge the gap, with platforms that record decentralized rating sessions for central quality control review, or even use AI to flag potential scoring inconsistencies.
Navigating the Challenges and Mitigating Risks in CNS DCTs
While the benefits are transformative, decentralized clinical trials for CNS are not without their own unique challenges that require careful planning and proactive navigation. Moving research from the controlled environment of a clinic into the variable setting of patients’ homes necessitates new strategies for ensuring data quality, technological equity, data security, and, above all, patient safety. These concerns, which have been highlighted in analyses by global bodies like European regulators, are entirely manageable with robust systems, thoughtful protocol design, and a steadfast commitment to patient support.
Addressing Technology and Data Integrity Concerns
Maintaining data integrity in a decentralized setting is a multi-faceted challenge that extends beyond the technology itself.
- Data Security and Privacy: Protecting highly sensitive CNS data (which can include genetic information, cognitive scores, and psychiatric evaluations) across multiple remote platforms is paramount. This requires end-to-end encryption, robust cybersecurity protocols, and strict compliance with data privacy regulations like GDPR and HIPAA.
- Platform and Data Integration: A typical DCT generates data from a wide array of sources: wearables, ePRO apps, telehealth platforms, and electronic health records (EHRs). These disparate data streams must be seamlessly ingested and integrated into a single, unified platform to provide investigators with a holistic view of the patient’s health.
- Validated Digital Tools: It is critical to use medical-grade, clinically validated digital health technologies. A consumer-grade fitness tracker may not have the accuracy or reliability needed to serve as a primary endpoint in a clinical trial. Sponsors must balance the need for scientific precision with the need for user-friendliness for the patient.
- User Training and Continuous Support: In a DCT, patients and their caregivers become active data collectors. They require clear, simple, and accessible training on how to use the technology. More importantly, they need access to a responsive, patient-centric technical support helpdesk that can quickly resolve issues to prevent data loss and patient frustration.
Bridging the Digital Divide and Ensuring Health Equity
A major risk in deploying technology-heavy trials is exacerbating health disparities. Reliance on personal smartphones or home internet can exclude older adults, individuals in rural areas with poor connectivity, and those from lower socioeconomic backgrounds. To ensure equitable access, sponsors must:
- Provide all necessary equipment: This includes provisioning pre-configured smartphones or tablets with a locked-down interface and a dedicated data plan, removing the technology and cost barrier for the participant.
- Design for digital literacy: User interfaces should be simple, intuitive, and available in multiple languages. Training materials should be offered in various formats (video, print, live support).
- Involve caregivers: For patients with significant cognitive or physical limitations, caregivers must be included in the technology training and support plan from the beginning.
Ensuring Patient Safety and Regulatory Compliance in a Remote Setting for Decentralized Clinical Trials for CNS
Patient safety remains the highest priority, and in a remote setting, it requires evolving oversight models and communication strategies.
- Remote Safety Monitoring: A robust plan for detecting and managing adverse events (AEs) in real-time is non-negotiable. This involves a clear workflow: an ePRO entry or sensor reading that crosses a pre-defined safety threshold could trigger an automated alert to a virtual site coordinator. The coordinator would then be responsible for contacting the patient (e.g., via video call) within a specified timeframe to assess the situation and escalate to the Principal Investigator (PI) as needed. This creates a system of continuous oversight.
- Investigator Oversight: The PI retains full responsibility for every patient’s safety, regardless of their location. Modern data platforms must provide PIs with a real-time dashboard to oversee all remote activities, monitor adherence, track safety signals across the entire study population, and intervene when necessary. This allows for more proactive oversight than waiting for the next scheduled visit.
- Regulatory and IRB Compliance: Protocols must be carefully designed to account for the unique aspects of DCTs. This includes processes for remote identity verification, secure electronic consent, managing direct-to-patient drug shipments, and navigating the complex web of state-by-state licensing requirements for telehealth providers and pharmacists.
- Clear and Redundant Communication: In an emergency, patients must know exactly who to contact and how. The trial must provide multiple, reliable communication channels (e.g., a 24/7 helpdesk phone number, secure in-app messaging, video call capabilities) that form the backbone of a safe and supportive remote trial experience.
Technology in a DCT must be used to improve and scale human oversight, not replace it. The human connection between the study team and the participant is the most critical component for ensuring patient safety and engagement.
Frequently Asked Questions about DCTs in CNS Research
What is the FDA’s official position on using decentralized elements in clinical trials?
The FDA supports decentralized clinical trials for CNS, especially after issuing guidance during the COVID-19 pandemic. Their position is based on three core principles:
- Patient Safety: Remote activities must not compromise participant well-being. Robust plans for monitoring adverse events are required.
- Data Integrity: Data collected remotely must be as reliable and accurate as data from a traditional site. This requires validated digital health technologies.
- Investigator Oversight: Principal Investigators retain ultimate responsibility for all trial activities, regardless of location.
The FDA has also provided practical recommendations for processes like electronic informed consent, encouraging DCT elements that improve patient access and data quality while maintaining scientific rigor.
How can sponsors ensure data quality from remote sources like wearables?
Ensuring high-quality data from remote sources requires the same rigor as traditional methods. Key strategies include:
- Use validated, medical-grade devices that are tested for accuracy in research settings.
- Develop standardized protocols with clear instructions for patients on device use and data collection.
- Implement central monitoring systems with anomaly detection to flag potential issues with data integrity or patient compliance. Platforms like Lifebit’s federated AI system can provide these real-time insights.
- Provide comprehensive rater training and ongoing calibration for any subjective assessments to minimize variability.
- Prioritize patient engagement with user-friendly interfaces and accessible technical support to ensure high compliance.
Are DCTs suitable for all types of CNS conditions?
No, decentralized clinical trials for CNS are not a one-size-fits-all solution, but their flexibility makes them adaptable to many conditions.
- Best suited for: Chronic conditions where travel is a significant burden (e.g., Parkinson’s, depression).
- Challenges with: Conditions requiring complex in-person assessments (e.g., advanced Alzheimer’s evaluations, specialized neuroimaging). Here, a hybrid model is invaluable, blending remote monitoring with essential on-site visits.
- Special considerations: Patients with significant cognitive impairment or motor skill limitations may require caregiver involvement and simplified digital tools.
The key is to design a trial that balances patient convenience with the scientific needs of the study, creating a custom hybrid model that works for a specific CNS condition and patient population.
Conclusion: The Future is Patient-Centric and Data-Driven
The era of site-centric research is ending, with decentralized clinical trials for CNS leading a patient-focused change. For the billion-plus people with CNS disorders, DCTs and hybrid models are breaking down barriers to participation, improving recruitment, retention, and data quality. While challenges in technology and security exist, they are being overcome with smarter design and stronger protocols.
The future of CNS research is not just decentralized; it’s data-driven. Artificial intelligence and machine learning are amplifying the power of DCTs, helping identify patients, analyze real-world data, and accelerate the path to new treatments. This is about empowering patients to contribute to breakthroughs on their own terms.
At Lifebit, we provide the federated AI infrastructure for this new era. Our platform, including our Trusted Research Environment (TRE) and R.E.A.L. (Real-time Evidence & Analytics Layer), enables secure, real-time analysis of global biomedical data while ensuring patient privacy and regulatory compliance.
The shift to decentralized, data-driven research is foundational. By embracing these approaches, we can ensure every patient has the opportunity to contribute to the medical breakthroughs of tomorrow.
Learn how to power your research with secure, real-time data analytics.

