The Ethics and Impact of Medical Research Data Sharing.

Medical research data sharing: Unlocking 2025
Why Medical Research Data Sharing Is Changing Healthcare
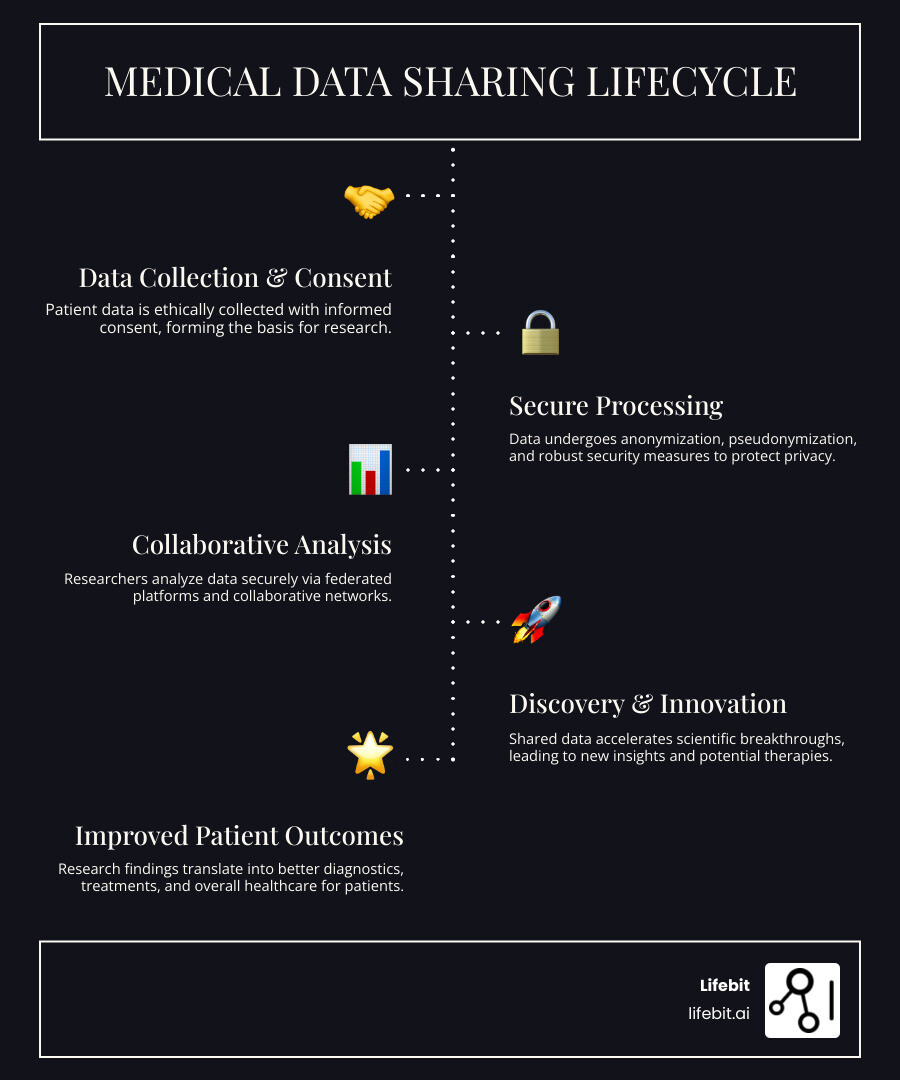
Medical research data sharing is a powerful force driving scientific breakthroughs and better patient outcomes. This shift toward open science is built on key principles: making data FAIR (Findable, Accessible, Interoperable, and Reusable), protecting patient privacy, ensuring scientific integrity, and fostering global collaboration under sound ethical governance.
The numbers tell a compelling story. Mentions of data sharing in PubMed articles grew over 20-fold between 1980 and 2019. This culture change is critical, as over 70% of researchers have struggled to reproduce experiments, highlighting the need for shared data to ensure research integrity.
This change isn’t just academic. Initiatives like the Pan-Cancer Analysis of Whole Genomes project brought together over 1,300 scientists from 37 countries. These collaborations prove that when researchers share data responsibly, patients get better treatments faster, and science moves forward more efficiently.
Yet challenges remain. More than half of the clinical trial data for approved cancer drugs is still inaccessible to independent researchers. Privacy regulations like GDPR add complexity, and researchers need proper credit for their data-sharing efforts.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. I’ve spent over 15 years developing computational biology tools and federated platforms that enable secure medical research data sharing. My work focuses on breaking down the barriers that prevent life-saving research collaborations from reaching their full potential.
Quick look at medical research data sharing:
- data intelligence platform
- ai drug findy platform
- cloud-based drug findy platforms market
Why Sharing Is Caring: The Benefits of Open Medical Data
When researchers share medical data, science accelerates, and patients benefit faster. Medical research data sharing acts as a powerful collaboration tool, turning every piece of information into a building block for new treatments. The benefits are clear: stronger scientific integrity, faster drug discovery, and a better return on research investment. By enabling data to be re-analyzed for new purposes, sharing maximizes the value of each participant’s contribution and every dollar spent on research. This approach not only prevents redundant studies but also opens the door to novel, cross-disciplinary insights that were impossible when data was locked away. Furthermore, studies show that sharing data leads to increased citation rates, a direct career incentive for researchers that helps align academic rewards with transparent practices.
This collaborative network effect is crucial for bridging the infamous “valley of death” between promising laboratory results and actual patient treatments. By pooling data, researchers can validate findings more quickly and build the robust evidence base needed to justify clinical trials, shortening the timeline from bench to bedside.
Tackling the Reproducibility Crisis
A sobering fact: more than 70% of researchers have failed to reproduce another scientist’s experiments. This reproducibility crisis is not just an academic concern; it has staggering economic consequences, with estimates suggesting that irreproducible preclinical research costs the U.S. economy approximately $28 billion annually. This waste of resources erodes public trust in science and delays medical progress. Medical research data sharing is a powerful antidote. By making raw data, analysis code, and methodologies available, it allows other scientists to verify the results independently. This transparent process helps catch honest errors, identify flawed methods, and ensure that findings are solid before they are used to inform clinical practice or subsequent research.
Beyond simple verification, open data enables powerful secondary analyses and meta-analyses. Researchers can combine datasets from multiple studies to increase statistical power, allowing them to detect effects that were invisible in smaller, individual studies. They can also ask entirely new questions of the data that the original investigators never envisioned. The Open Science movement is gaining significant momentum to foster this environment, with over 1,000 scientific journals now encouraging or requiring data sharing. This transparency also directly combats publication bias—the tendency for journals to publish positive or novel results while negative or null findings are ignored. Sharing all data, regardless of the outcome, leads to a more complete and honest scientific record, preventing other researchers from wasting time and money pursuing avenues that have already been proven ineffective. For more insights on how sharing data boosts research impact, check out the citation advantage of sharing data.
Fueling Precision Medicine and Faster Cures
The future of medicine is personal. Precision medicine aims to tailor therapies to a patient’s unique genetic makeup, lifestyle, and environment. This approach relies on training sophisticated Artificial Intelligence (AI) and machine learning algorithms on vast, diverse datasets. These powerful tools can spot subtle patterns in complex biological data, predict individual responses to treatments, identify novel biomarkers for early disease detection, and pinpoint new targets for drug development. However, these algorithms are only as good as the data they learn from.
To be effective and equitable, AI models require data from large and globally diverse populations. Training on limited or homogenous datasets can lead to biased algorithms that perform poorly for underrepresented groups, exacerbating health disparities. Large-scale projects show what is possible when data is shared responsibly. The UK Biobank, with its deep genetic and health data from 500,000 volunteers, has become a cornerstone of biomedical research. Similarly, the 1+ Million Genomes Initiative in Europe and the Pan-Cancer Analysis of Whole Genomes project (involving over 1,300 scientists from 37 countries) have created unparalleled resources for understanding disease at a molecular level. These international genomics collaborations prove that Big Data analytics becomes exponentially more powerful when it draws from a rich tapestry of global populations.
At Lifebit, we’ve seen firsthand how federated AI platforms can securely analyze global biomedical data in real-time. By bringing the analysis to the data, these platforms turn months of logistical and analytical work into hours of insight, dramatically accelerating the journey from research to cure.
For a deeper dive into how big data is changing healthcare, explore More on Big Data to Precision Medicine.
The Ethical Tightrope: Balancing Progress with Patient Privacy
Medical research data sharing requires a delicate balance: using data to save lives while rigorously protecting the privacy of the individuals who entrust us with their most sensitive information. Patient trust is the bedrock of medical research, and it is fragile. High-profile incidents involving the misuse of health data have shown that good intentions are not enough. To maintain public confidence and ensure the long-term viability of data-driven research, we need rock-solid technical safeguards, transparent governance, and a deep commitment to the principle of data dignity—respecting individuals and their data at every step.
The challenges of data misuse, unauthorized access, and re-identification are real. A single breach can have devastating consequences for individuals and set back public willingness to participate in research for years. Yet the potential to prevent and cure diseases makes this ethical tightrope worth walking, provided we do so with the utmost care and respect.

Consent, Anonymization, and Data Security
Ethical data sharing begins with informed consent. Traditional, one-time consent models are often inadequate for the dynamic nature of modern research. New models are emerging:
- Broad Consent: Allows patients to consent to their data being used for a wide range of future research projects, which is efficient but offers less patient control.
- Tiered Consent: Gives patients a menu of options, allowing them to approve certain types of research (e.g., non-profit academic research) but not others (e.g., commercial research).
- Dynamic Consent: This patient-centered approach, explored in this viewpoint on personalized consent flow, uses digital platforms to maintain an ongoing dialogue with participants, allowing them to manage their preferences and receive updates on how their data is contributing to science.
Data anonymization, which removes personally identifiable information (PII), is a cornerstone of privacy protection. However, true anonymization is technically challenging with rich medical datasets. Seemingly innocuous data points (like zip code, date of birth, and gender) can act as quasi-identifiers, which, when combined, can be used to re-identify individuals. This has led to a greater reliance on pseudonymization, where direct identifiers are replaced by a code.
Protecting this data requires a multi-layered approach to security. Data stewardship—the responsible management of data throughout its lifecycle—is critical. This is supported by strong data security protocols that go beyond basic encryption and access controls. A robust security framework includes end-to-end encryption for data both in transit and at rest, strict role-based access controls, comprehensive audit trails to log all data access and activity, and physical security for servers. These measures are essential for creating secure research environments where data can be used responsibly.
Navigating Global Regulations like GDPR and HIPAA
The legal landscape for data sharing is a complex maze of national and international rules. The EU’s General Data Protection Regulation (GDPR) set a new global standard in 2018, fundamentally shifting the focus to individual data rights. For researchers, GDPR introduces key concepts like the legal basis for processing data, the roles of Data Controller and Data Processor, and stringent requirements for documenting data protection measures. It grants individuals powerful rights, including the right to access their data, the right to rectification, and the right to erasure (the “right to be forgotten”). Fines for non-compliance can be severe, reaching up to 4% of global annual turnover.
Other key regulations create a patchwork of requirements globally:
- HIPAA in the U.S. governs the use and disclosure of Protected Health Information (PHI). It outlines specific methods for de-identification, such as the Safe Harbor method, which involves removing 18 specific identifiers.
- CCPA (California Consumer Privacy Act), now expanded by the CPRA, grants California residents rights similar to those under GDPR.
- PIPEDA in Canada’s private sector governs how organizations collect, use, and disclose personal information.
- Emerging laws like Brazil’s LGPD and Japan’s APPI are creating similar data protection frameworks worldwide.
Cross-border data transfer remains a major hurdle, as conflicting legal requirements can slow down or halt vital international research projects. Harmonizing data governance frameworks and developing technical solutions that allow for collaboration while respecting jurisdictional boundaries is one of the most significant challenges in modern medicine, requiring innovative approaches that protect privacy without stifling progress.
The Current Landscape of Medical Research Data Sharing
For decades, valuable medical data has been trapped in silos. This fragmentation—with data locked away in incompatible hospital EMR systems, proprietary pharmaceutical company vaults, and isolated academic research databases—has been a major barrier to breakthroughs. These silos form due to a combination of technical hurdles, a lack of data standards, institutional policies prioritizing control over collaboration, and commercial interests. However, a fundamental shift is underway as stakeholders across the healthcare ecosystem realize that in the fight against disease, collaboration beats competition.

Perspectives from Patients, Industry, and Academia
- Patients are increasingly active partners in research, not just subjects. Driven by altruism and a desire for cures, many are strong supporters of data sharing, as exemplified by the success of programs like the All of Us Research Program. Patient advocacy groups are powerful catalysts, championing the “Nothing About Us Without Us” principle and demanding transparency, control, and a seat at the table in data governance.
- Pharmaceutical companies, traditionally protective of their data for competitive reasons, are increasingly engaging in pre-competitive collaboration. Consortia like the Innovative Medicines Initiative (IMI) and TransCelerate BioPharma bring rivals together to share data and resources to tackle common challenges in drug development. However, gaps remain; a 2021 study found that over 50% of clinical trials for approved cancer drugs in the past decade still had data inaccessible to independent researchers. Progress toward full transparency is inconsistent.
- Academic institutions are at the heart of the tension between the traditional “publish or perish” culture and the push for open science. While researchers have been hesitant to share data for fear of being “scooped,” this is changing. Major funding bodies like the NIH in the U.S. and the Wellcome Trust in the U.K. now mandate data management and sharing plans in grant applications, creating a powerful incentive for change. Universities are developing new policies to formally recognize and reward researchers for sharing well-curated datasets.
- Regulatory bodies like the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are championing transparency. They mandate the registration of clinical trials and the publication of summary results on public registries like ClinicalTrials.gov. However, policies requiring the proactive sharing of individual participant-level data (IPD) are still evolving and not yet universal.
A Look at Data Sharing Platforms and Publisher Policies
The infrastructure for medical research data sharing has grown dramatically. Centralized repositories like the NIH’s dbGaP (Database of Genotypes and Phenotypes) and the European Genome-phenome Archive (EGA) host vast amounts of genomic and health data, accessible to approved researchers. Portals like ClinicalStudyDataRequest.com and Vivli provide a streamlined process for requesting access to clinical trial data from multiple industry sponsors.
Technology is also evolving beyond simple data repositories. The most significant shift is toward Trusted Research Environments (TREs), also known as data safe havens, and Trusted Data Lakehouses (TDLs). A TRE is a highly secure computing environment where sensitive data is held. Approved researchers are given access to the environment and analytical tools but cannot download the raw data itself. All analyses happen within the secure perimeter, and only approved, non-identifiable results can be exported. This model, which our platform components are built on, maximizes data utility for research while minimizing the risk of a data breach, enabling secure analysis of global biomedical data.
Publishers are also key drivers of change. The influential International Committee of Medical Journal Editors (ICMJE) requires authors to include a data sharing statement with their manuscript submissions. Furthermore, the Transparency and Openness Promotion (TOP) guidelines, which provide a framework for journal policies on data, code, and materials sharing, have been adopted by over 1,000 journals, signaling a broad cultural shift in scientific publishing.
For an overview of how modern platforms enable secure collaboration without compromising privacy, you can learn more about secure data collaboration solutions.
Pioneering the Future of Data Collaboration
The future of medical research data sharing is not about creating one giant, centralized database. It’s about building secure, intelligent bridges that allow insights to flow between distributed datasets while ensuring patient privacy and institutional governance are respected. This is being achieved through a combination of smart technology, robust standards, and new paradigms for handling sensitive information. Instead of hoarding data or risking its movement, we are creating a federated ecosystem where the data remains safe and the insights travel.

Best Practices for Medical Research Data Sharing: The FAIR Principles and Academic Recognition
The FAIR Guiding Principles have become the globally recognized gold standard for data management, ensuring that data is fit for sharing and reuse by both humans and machines. You can dive deeper into the FAIR Guiding Principles. FAIR stands for:
- Findable: For data to be reused, it must first be found. This means assigning it a globally unique and persistent identifier (like a Digital Object Identifier, or DOI) and describing it with rich metadata that clearly explains its content, origin, and context. This metadata should be indexed in a searchable resource.
- Accessible: Once found, data should be accessible. This doesn’t necessarily mean ‘open.’ It means that the protocols for accessing the data—including authentication and authorization—are clearly defined. Access may be restricted and require review by a data access committee, but the process for requesting access should be transparent.
- Interoperable: Data needs to be able to work with other datasets and with applications for analysis. This requires the use of common data models (like the OMOP Common Data Model), standardized vocabularies, and ontologies (such as SNOMED CT for clinical terms or LOINC for lab tests). This semantic interoperability is key to integrating diverse data sources.
- Reusable: The ultimate goal is to make data reusable for future research. This requires rich documentation, including a clear data license, detailed information on data provenance (its history and lifecycle), and the study protocols used to generate it. Without this context, a dataset is just numbers; with it, it becomes a valuable scientific asset.
Making data FAIR takes significant effort. To incentivize this crucial work, the academic community is adopting new recognition systems. Data citation standards allow datasets with DOIs to be cited just like research papers, and metrics like the S-Index are emerging to measure the impact of shared data, ensuring researchers receive formal credit for their contributions.
Innovations in Medical Research Data Sharing: Federated Approaches and Beyond
The most game-changing innovation in secure data sharing flips the traditional model on its head: instead of moving data to a central location for analysis, we bring the analysis to the data. This is the principle behind federated data analysis. In this model, an analytical query is sent to multiple datasets in their respective secure environments. Each dataset is analyzed locally, behind its own firewall. Only the aggregated, non-sensitive results are returned to the researcher. This approach, which our federated AI platform enables, allows for powerful, real-time analysis of global biomedical data without the sensitive information ever leaving its protected location.
Other key privacy-enhancing technologies (PETs) are maturing:
- Privacy-Preserving Computation: This includes techniques like homomorphic encryption, which allows for calculations to be performed directly on encrypted data, and secure multi-party computation, where multiple parties can jointly compute a function over their inputs without revealing those inputs to each other.
- Differential Privacy: A mathematical framework for quantifying privacy risk. It involves adding carefully calibrated statistical noise to query results, making it impossible to determine whether any single individual’s data was included in the analysis, thus protecting privacy while preserving the accuracy of aggregate insights.
- Synthetic Data Generation: This involves creating artificial datasets that mimic the statistical properties and patterns of the original sensitive data. Researchers can then work freely with the synthetic data for model development and exploration, as it contains no real patient information.
- Patient-Centric Sharing Models: Initiatives like Sync for Science empower patients to use APIs to download their health records and direct them to researchers, giving them granular control over their own information.
These technologies are no longer theoretical; they are being deployed now, making medical research data sharing more secure, collaborative, and powerful than ever.
Frequently Asked Questions about Medical Research Data Sharing
Here are answers to common questions about medical research data sharing.
What is the difference between anonymized and pseudonymized data?
Understanding this distinction is crucial for privacy and compliance with regulations like GDPR.
- Anonymized data has had all identifying information permanently removed, so an individual cannot be re-identified. True anonymization is difficult with complex medical data and can reduce its research utility. Under GDPR, it is no longer considered “personal data.”
- Pseudonymized data replaces direct identifiers (like a name) with a code. A secure key, stored separately, allows for potential re-identification. This data is still considered “personal data” under GDPR but strikes a balance between privacy and research value, making it common in medical research.
How can researchers get credit for sharing their data?
Recognizing data creators is essential. The academic culture is shifting to reward data sharing in several ways:
- Data citation standards allow datasets to be assigned a Digital Object Identifier (DOI), making them citable just like a publication.
- The S-Index is an emerging metric that measures the impact of a researcher’s shared data.
- Journal and funder mandates increasingly require data sharing statements and plans, integrating this practice into the academic reward system.
Are patients generally supportive of their data being used for research?
Yes, but with important conditions. Most patients are willing to share their data, often driven by altruism. However, this support depends on:
- Trust: Patients must have confidence that institutions will protect their data.
- Transparency: Clear communication about how data will be used, by whom, and with what safeguards is essential.
- Control: Patients increasingly prefer dynamic consent models, which give them ongoing choice over how their data is used, rather than one-time blanket permission.
When these conditions are met, patient participation can be very high.
Conclusion
The world of medical research data sharing is at an exciting crossroads. We’ve seen how responsible sharing accelerates drug findy, enables precision medicine, and builds more trustworthy science, as demonstrated by massive collaborations like the Pan-Cancer project. This progress is driven by patients who generously share their data and researchers dedicated to collaboration.
The challenges are significant, from balancing progress with patient privacy to navigating complex global regulations like GDPR. Ensuring everyone in the ecosystem feels valued and protected requires constant effort.
Yet the future is promising. Privacy-enhancing technologies like federated learning are revolutionizing secure analysis, while the FAIR Principles are creating universal standards for data utility.
At Lifebit, we are building the technical bridges for this new era. Our federated AI platform enables researchers to collaborate with global biomedical data while keeping it secure and compliant, without compromising privacy or governance.
The road ahead requires partnership. By working together with transparency and respect for privacy, medical research data sharing becomes more than a technical challenge—it becomes a powerful force for healing.
Ready to explore how secure data collaboration can accelerate your research? Learn more about our secure data collaboration solutions and join us in building a healthier future for everyone.

