Who’s Who in Real-World Data: A Look at Top Companies and Services
Real World Data Company: Smart Insights 2025
The Growing Landscape of Real-World Data Companies
A real world data company specializes in collecting, managing, and analyzing healthcare data from everyday clinical practice to generate evidence for better healthcare decisions. Here are the main types of real-world data companies you’ll encounter:
Top Real-World Data Company Categories:
- Centralized Platforms – Store massive datasets (1B+ patient records) for broad analysis
- Federated Networks – Enable secure analysis across healthcare organizations without moving data
- Specialty Providers – Focus on specific diseases like oncology or rare conditions
- Data Connectivity – Link different data sources while protecting patient privacy
- Analytics Platforms – Transform raw data into actionable healthcare insights
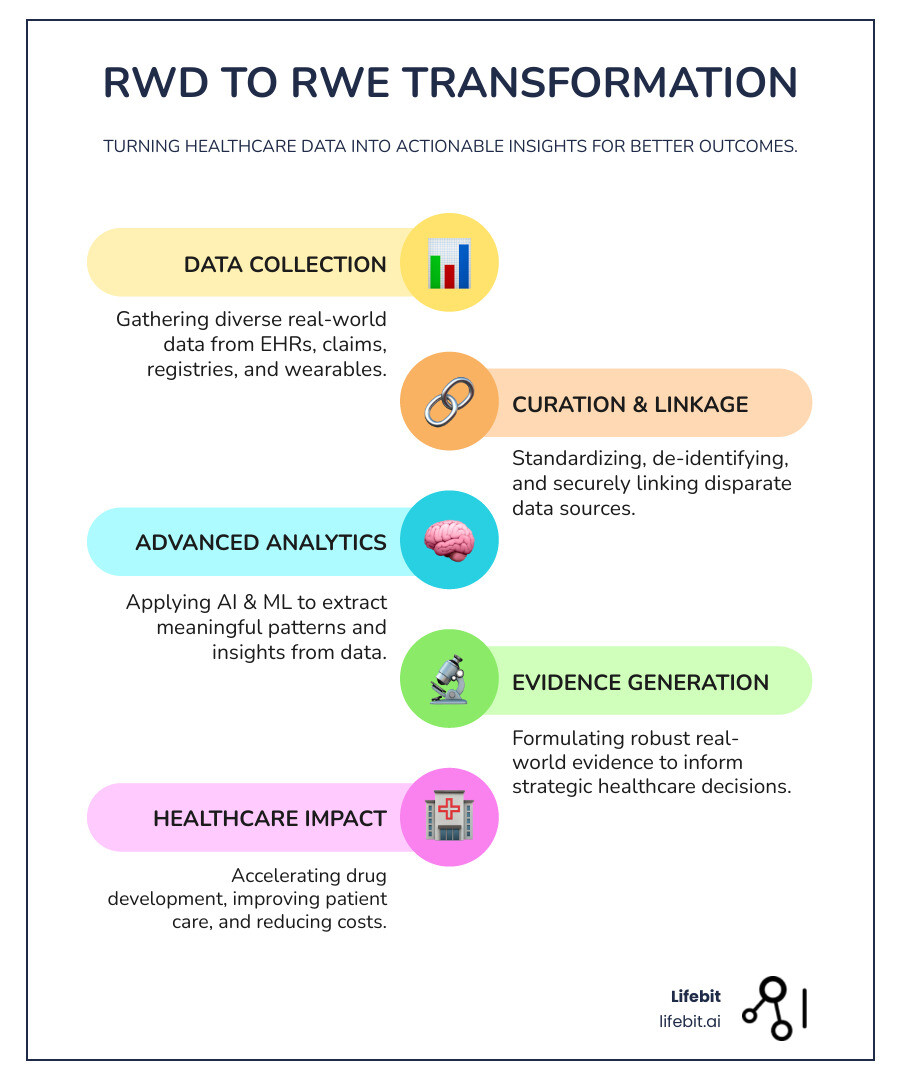
The healthcare industry faces a striking challenge: 97% of data generated by the healthcare sector goes unused. This represents billions of dollars in lost opportunities and countless missed chances to improve patient outcomes. Real-world evidence (RWE) platforms have emerged as the solution, changing raw healthcare data into actionable insights that drive better clinical decisions.
Real-world data encompasses information collected from everyday healthcare delivery – electronic health records, insurance claims, patient registries, and even wearable devices. When properly analyzed, this data becomes real-world evidence that can accelerate drug development, improve patient care, and reduce healthcare costs.
The RWE landscape includes diverse company models, each serving different needs across the pharmaceutical and healthcare ecosystem. Some focus on massive scale and breadth, others on deep specialty expertise, and still others on innovative federated approaches that keep data secure while enabling powerful analytics.
As CEO and Co-founder of Lifebit, I’ve spent over 15 years working at the intersection of computational biology, AI, and health-tech entrepreneurship, helping organizations open up the potential of biomedical data through federated analysis platforms. My experience building tools for precision medicine has given me deep insight into how different real world data company models serve the evolving needs of global healthcare organizations.
Real world data company word list:
The Foundation: What is Real-World Data and Why is it Significant?
Think of real-world data as the digital breadcrumbs that patients and healthcare providers leave behind during everyday medical care. Unlike the carefully controlled environment of clinical trials, real-world data (RWD) captures what actually happens when patients receive treatment in hospitals, clinics, and even at home.
The U.S. Food and Drug Administration defines RWD as information about patient health status and healthcare delivery that’s routinely collected from various sources. This data tells the complete story of a patient’s healthcare journey – not just the highlights captured in traditional research studies.
The main sources of RWD paint a comprehensive picture of healthcare:
Electronic Health Records (EHRs) serve as the digital backbone of modern healthcare, containing everything from medical histories and diagnoses to lab results and treatment notes. Claims data reveals the financial side of healthcare – what services were provided, which procedures were performed, and what medications were prescribed. Disease registries create organized collections of patient information for specific conditions, while wearables and digital health devices capture real-time data about how patients feel and function in their daily lives.
Social Determinants of Health (SDoH) add another crucial layer, showing how factors like housing, education, and income affect health outcomes. For healthcare organizations wanting to dive deeper, our comprehensive real-world data definition explores these concepts in greater detail.
Why does this matter so much? Traditional clinical trials, while essential, only show us part of the picture. They typically involve carefully selected patients in controlled settings – which doesn’t always reflect how treatments work for diverse populations in the real world.
Real-world data changes this by helping us understand diverse populations that might be underrepresented in clinical trials. It allows us to assess cost-effectiveness by tracking actual resource use and outcomes, not just theoretical projections. Most importantly, it enables data-driven decision-making across the entire healthcare ecosystem.
The real magic happens when raw RWD transforms into Real-World Evidence (RWE). This is where a real world data company adds tremendous value – taking messy, unstructured healthcare data and applying sophisticated analytical methods to generate reliable insights.
These insights inform critical decisions for regulators evaluating drug approvals, payers determining reimbursement policies, and healthcare providers personalizing treatments for their patients. It’s the difference between having a pile of puzzle pieces and seeing the complete picture they create when properly assembled.
The Architects of Insight: A Guide to Real-World Data Company Models

The landscape of real world data company models is as diverse as the data itself. Each model offers unique strengths, catering to different needs across the life sciences value chain, from early-stage research to post-market surveillance. While we won’t name specific companies, we’ll explore the categories that define the leaders in this space.
Centralized Data Platforms: Breadth and Scale
These organizations act as custodians of immense, secure repositories of de-identified patient data. Their strength lies in the sheer volume and breadth of data they control, often integrating information from millions of data feeds and billions of non-identified patient records. Imagine a digital ocean of health information, constantly updated and carefully curated.
These platforms typically offer end-to-end solutions, helping life science companies demonstrate the value of their products, inform commercial strategies, and optimize market access. We’ve seen how these large-scale datasets can be invaluable for conducting broad epidemiological studies or sizing potential markets for new therapies. For instance, understanding the real-world impact on pricing and reimbursement is crucial, and these platforms provide the data to inform such complex decisions. You can learn more about RWD’s impact on price & reimbursement in our detailed article.
Federated Network Solutions: Collaboration and Privacy
In contrast to centralized models, federated networks emphasize collaboration and privacy by allowing secure analysis across multiple healthcare organizations without moving the underlying data. The data remains at its source, within the healthcare provider’s secure environment. A federated real world data company provides the technology and governance framework that enables researchers to query and analyze these distributed datasets in real-time, receiving aggregated results without ever seeing individual patient data.
This model is particularly powerful for clinical trial feasibility and site selection. Imagine needing to identify patient cohorts for a clinical trial across a vast network of hospitals in different countries. A federated network can query these diverse sources, encompassing data from over 90 healthcare organizations in 20+ countries, covering tens of millions of patient lives, and providing up to 10 years of clinical history. This capability streamlines the process significantly. We’ve explored the immense benefits of real-world data in clinical research extensively.
Specialty-Focused Approaches: Depth and Curation
Some real-world data companies carve out a niche by specializing in deep, highly curated datasets for specific diseases or therapeutic areas. This often includes complex conditions like oncology, rare diseases, or neurology, where granular, patient-level insights are paramount.
Their focus isn’t just on volume, but on the richness and quality of the data. This means carefully processing unstructured data, such as clinician notes and pathology reports, and integrating multi-omic data like genomic and biomarker information. This deep dive allows for precise target identification for new therapies and comprehensive natural history studies to understand disease progression. Our insights on using RWE for target identification highlight the power of this approach. For example, studying the progression of a rare cancer might involve combining detailed genomic profiles with longitudinal clinical records to understand every nuance of the disease.
Data Connectivity & Tokenization Enablers
A specialized type of real world data company focuses on the crucial task of linking disparate datasets while carefully protecting patient privacy. They are the architects of interoperability in the RWD ecosystem. Their core technology involves de-identification and tokenization, which replace sensitive patient identifiers with unique, anonymous codes. This allows different datasets – say, a patient’s clinical trial data and their long-term outcomes from insurance claims – to be securely linked without compromising privacy.
These enablers are vital for building a truly linkable data infrastructure, helping to overcome the pervasive data silos that plague the healthcare sector. We’ve seen significant shifts in the industry towards tokenization, with some platforms reporting a remarkable 300% growth in the volume of trials being tokenized. This innovation helps augment existing datasets, providing a more complete picture of patient journeys and treatment effectiveness.
Core Capabilities of a Modern Real-World Data Company
The healthcare data landscape has evolved dramatically, and today’s real world data company needs to be much more than just a data warehouse. Technology has become the ultimate differentiator, moving far beyond simply providing access to information. The real magic happens in the platform capabilities that transform mountains of raw healthcare data into insights that can actually change patient lives.
Think of it this way: having access to billions of patient records is like owning a massive library. But without the right tools to search, analyze, and understand all those books, you’re essentially sitting in a very expensive storage room. Modern RWD platforms are the librarians, search engines, and speed-readers all rolled into one sophisticated system.
The Power of AI and Machine Learning in RWD
Artificial Intelligence and Machine Learning have become the powerhouse engines driving real value from vast RWD collections. These aren’t just fancy buzzwords – they’re solving real problems that would be impossible to tackle manually.
Pattern recognition capabilities allow AI algorithms to spot subtle connections and correlations across massive datasets that even the most experienced human analysts would miss. We’re talking about finding needles in haystacks the size of entire cities. These patterns often lead to breakthrough findings that can redirect entire research programs.
Predictive modeling takes things a step further, using ML models to forecast disease progression, predict how patients will respond to specific treatments, and even flag patients who might be at risk for certain conditions before symptoms appear. It’s like having a crystal ball, but one backed by rigorous science and massive amounts of real-world data.
Perhaps most impressive is Natural Language Processing (NLP), which tackles one of healthcare’s biggest challenges: all that unstructured text hiding in physician notes, pathology reports, and clinical narratives. NLP can read through millions of doctor’s notes and extract meaningful, structured insights that would otherwise remain locked away in free-text fields.
The real-world benefits are remarkable. AI and ML accelerate analysis from months to weeks, help identify completely novel drug targets, and dramatically improve our ability to predict patient outcomes. For instance, machine learning algorithms can analyze EHR data to identify patients who might be misdiagnosed, enabling earlier interventions and potentially saving lives. Our deep dive into optimizing real-world evidence in pharma explores these applications in much greater detail.
Ensuring Data Privacy, Security, and Compliance
Here’s where things get tricky – and absolutely critical. The healthcare industry deals with some of the most sensitive personal information imaginable, and the challenges around privacy, security, and regulatory compliance are enormous. It’s like trying to build a fortress that’s simultaneously completely secure and easily accessible to authorized researchers worldwide.
Navigating complex global regulations while maintaining patient trust requires walking a very careful tightrope. Every real world data company must balance the incredible potential of health data with the fundamental responsibility to protect patient privacy. We’ve written extensively about the challenges of using real-world data in research, and these challenges are both technical and ethical.
Leading platforms address these challenges through multiple layers of protection. Data encryption ensures information is protected both when it’s stored and when it’s moving between systems. Access controls create detailed permissions systems, ensuring only authorized personnel can access specific datasets for approved purposes.
Federated governance establishes comprehensive rules and oversight frameworks for how data can be accessed and used across distributed networks. This is particularly important when dealing with international collaborations where different countries have different privacy laws.
De-identification and tokenization represent some of the most sophisticated privacy-protecting technologies available. These approaches anonymize patient data by removing personal identifiers while still allowing researchers to link and analyze information across different sources.
The regulatory landscape continues to evolve rapidly, with frameworks like HIPAA in the United States and GDPR in Europe setting increasingly stringent standards. Our analysis of US regulatory guidance on using real-world data shows just how important it is to stay ahead of these requirements. Compliance isn’t just about avoiding legal trouble – it’s fundamental to building and maintaining the trust that makes this entire ecosystem possible.
From Data to Evidence: Analytics and Visualization
Once data is cleaned, secured, and ready for analysis, the real fun begins. Modern analytics platforms feature user-friendly interfaces with intuitive query builders that let researchers explore and analyze data on demand. This “self-service” approach is transformative – instead of waiting weeks or months for IT teams to run custom queries, scientific teams can generate insights in real-time.
Think of it like the difference between having to ask a librarian to find every book for you versus being able to browse the shelves yourself. The speed and flexibility this provides can completely transform how research gets done.
Data visualization tools are equally crucial, changing complex datasets into digestible dashboards and compelling reports. Imagine being able to simulate entire healthcare pathways or visualize patient journeys through interactive charts and graphs. These tools don’t just make insights more accessible – they help identify trends and patterns that might not be obvious in raw data tables.
The ultimate goal is empowering researchers and data scientists to move quickly from questions to answers. Modern platforms can simulate healthcare pathways, create dynamic dashboards, and generate publication-ready reports – all while ensuring that every step of the analysis maintains the highest standards for data privacy and scientific rigor.
The Impact: How RWD Transforms Drug Development and Patient Care
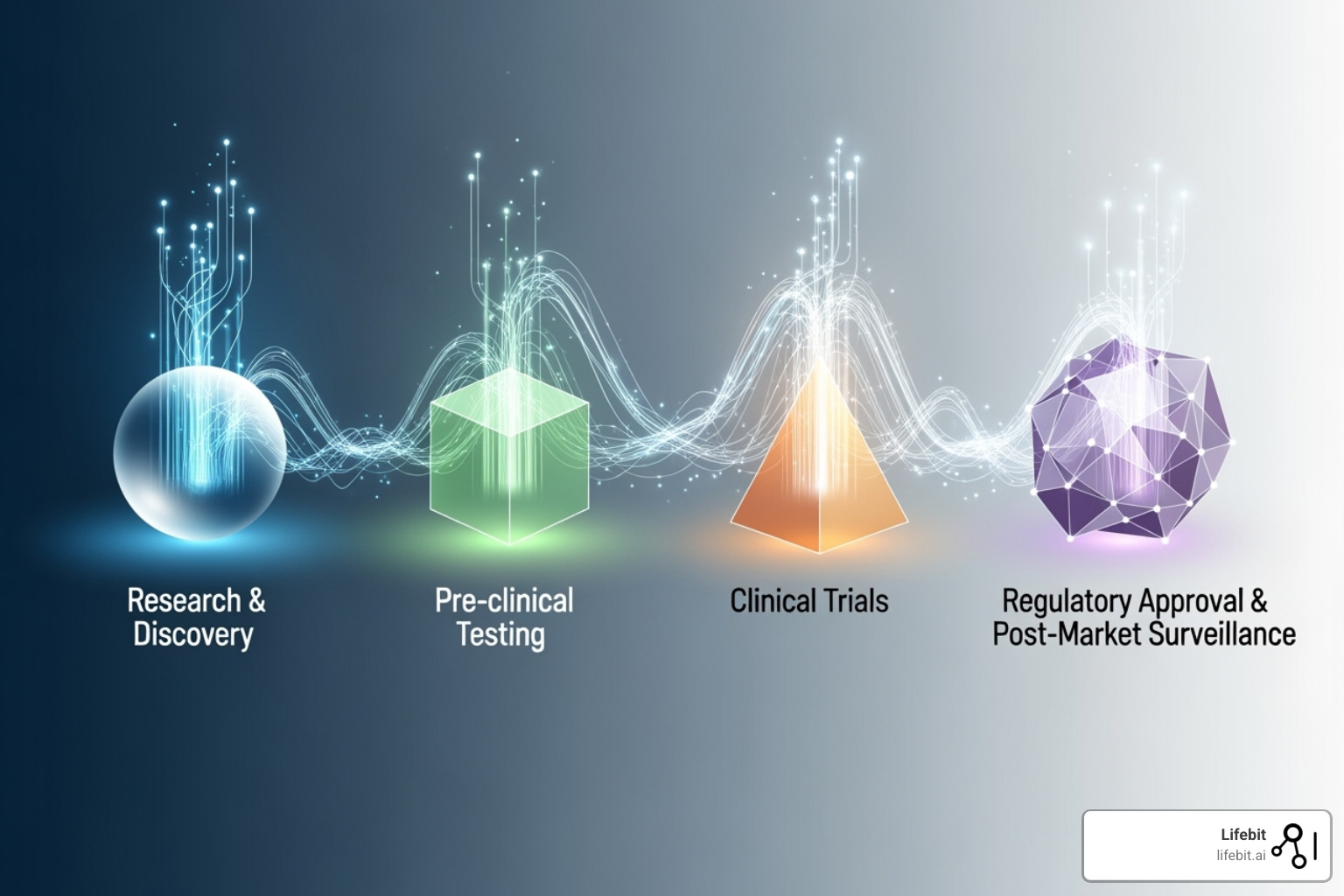
Healthcare is experiencing a fundamental shift. Where we once relied on limited clinical trial data and educated guesswork, we now have the power to make data-driven decisions based on real-world evidence from millions of patients. This change touches every aspect of healthcare, from the earliest moments of drug discovery to the ongoing care of patients worldwide.
The impact of this change cannot be overstated. Every real world data company is contributing to a revolution that’s making healthcare more effective, more accessible, and more personalized than ever before.
Accelerating the Entire Drug Development Lifecycle
Real-world data is reshaping drug development from start to finish, creating opportunities that simply didn’t exist a decade ago.
In the early stages of development, RWD helps researchers identify promising drug targets by revealing hidden patterns in disease progression and highlighting unmet medical needs. Instead of starting with theoretical assumptions, teams can begin with solid evidence about what patients actually experience. This foundation also improves clinical trial design by helping researchers identify the right patient populations and optimize protocols – potentially saving hundreds of millions of dollars in development costs.
During clinical trials, the benefits become even more tangible. RWD streamlines patient recruitment by efficiently identifying eligible participants across vast healthcare networks. Perhaps more importantly, it enables the creation of external control arms, reducing the need for placebo groups in certain studies. This means trials can move faster while still maintaining scientific rigor. Our detailed exploration of RWD in clinical trials shows just how transformative this approach can be.
The value continues well into the post-market phase. After a drug receives approval, RWD becomes essential for pharmacovigilance and safety surveillance. Instead of waiting for adverse events to be reported through traditional channels, researchers can continuously monitor safety signals across diverse patient populations. This real-time insight also supports label expansion studies and helps demonstrate the long-term value of therapies as they’re used in everyday clinical practice.
Addressing Unmet Needs and Improving Patient Outcomes
Real-world data excels at uncovering the gaps in healthcare that matter most to patients.
Understanding disease becomes far more nuanced when we can conduct comprehensive natural history studies using real-world data. These studies reveal how diseases actually progress in diverse populations, not just in the carefully controlled environments of clinical trials. They help identify specific patient subgroups who may respond differently to treatments, opening doors to more personalized approaches to care.
Improving access to life-saving treatments often comes down to demonstrating value to payers and insurance companies. Real-world evidence provides the proof that treatments work in everyday practice, not just in ideal clinical settings. This evidence is crucial for pricing and reimbursement negotiations, ensuring that effective therapies reach the patients who need them most.
The move toward patient-centricity is perhaps the most exciting development. We’re now incorporating patient-generated data from wearables and digital health tools directly into our evidence generation. This trend is accelerating rapidly – more than 1 in 2 consumers now track their health with digital tools, and about 43% own a wearable device, according to ROCK HEALTH.
This wealth of patient-generated data provides a complete picture of how treatments affect people’s daily lives, not just their clinical markers. It empowers researchers to understand patient preferences and experiences in ways that were impossible before, leading to care that’s truly centered around what matters most to patients.
The Future of RWE and Emerging Trends for a Leading Real World Data Company
The healthcare industry stands at an exciting crossroads. We’re witnessing growing regulatory acceptance of real-world evidence from the FDA, EMA, and other global authorities, while payers increasingly rely on RWE to make reimbursement decisions. This shift represents a fundamental change in how we approach healthcare innovation.
The data landscape itself is expanding rapidly. Genomics and proteomics are becoming integral parts of the RWD ecosystem, allowing us to understand diseases at the molecular level like never before. Meanwhile, digital biomarkers from wearables are providing continuous, passive monitoring that captures the 90% of patient data we traditionally missed between clinical visits.
What’s particularly exciting is how technology is solving the complex challenges that once seemed impossible. Federated AI and learning now allows artificial intelligence models to be trained across multiple datasets without the data ever leaving its secure home. This means we can harness the power of global health data while maintaining the highest privacy standards.
Privacy-enhancing technologies (PETs) are revolutionizing how we handle sensitive health information. These innovations enable sophisticated analysis while keeping individual patient data completely protected – it’s like having your cake and eating it too.
The shift toward cloud-first strategies is equally transformative. Cloud platforms are providing scalable, flexible environments that make RWD analysis faster and more accessible to researchers worldwide. This democratization of data access is accelerating findies across the entire healthcare ecosystem.
Looking ahead, the most successful real world data company will focus on three key areas. More integrated evidence generation will seamlessly combine traditional clinical trial data with real-world insights, giving us a complete picture of how treatments perform. Proactive safety monitoring powered by AI will detect potential issues months or even years earlier than current methods allow.
Perhaps most importantly, we’re moving toward personalized medicine at scale. By leveraging deep RWD insights, we can tailor treatments to individual patients based on their unique genetic profiles, medical history, and real-world responses to therapy.
The path forward isn’t without challenges, but the technological solutions are keeping pace. Our comprehensive guide on 7 steps for RWD outlines how organizations can steer this evolving landscape effectively.
The future of healthcare is data-driven, collaborative, and patient-centric. As these trends continue to mature, we’re not just improving how we develop drugs – we’re fundamentally changing how we understand and treat human disease.
Conclusion: Partnering for Success in the RWE Landscape
The healthcare landscape is undergoing a profound change, and real world data company models are at the heart of this change. We’ve explored how different approaches serve unique purposes – from centralized platforms that harness the power of billions of patient records to federated networks that enable secure collaboration without compromising privacy. Specialty providers deliver the deep, curated insights needed for complex conditions, while connectivity enablers break down data silos to create a more complete picture of patient health.
The beauty of this diverse ecosystem is that there’s no one-size-fits-all solution. Your research question drives everything. Are you studying a rare disease that affects thousands of patients globally? You might need the deep, specialized datasets that specialty-focused companies provide. Planning a large-scale epidemiological study? The breadth and scale of centralized platforms could be your answer. Need to analyze data across multiple healthcare organizations while keeping sensitive information secure? A federated approach might be perfect.
The key is understanding what you’re trying to achieve and matching that with the right technological capabilities. Some projects require the ability to process unstructured clinical notes using advanced AI. Others need real-time querying across distributed datasets. Still others demand the highest levels of privacy protection while enabling meaningful analysis.
As the industry continues to evolve, we’re seeing these capabilities converge. Next-generation platforms are integrating the best of all worlds – offering federated AI to securely analyze sensitive biomedical and multi-omic data in-place. This approach powers research at scale while maintaining the privacy and security that healthcare data demands.
At Lifebit, we’ve built our platform around this vision of integrated capabilities. We enable large-scale, compliant research and pharmacovigilance across biopharma, governments, and public health agencies. Our federated approach delivers real-time insights and enables secure collaboration across hybrid data ecosystems – all while keeping sensitive data exactly where it belongs.
The future of healthcare depends on our ability to transform the vast amounts of data we generate every day into actionable insights. Whether you’re developing the next breakthrough therapy, monitoring drug safety, or working to improve patient outcomes, the right real world data company partnership can accelerate your mission and amplify your impact.
Ready to explore how federated technology can transform your approach to real-world evidence? Learn how federated technology can accelerate your RWE strategy and join the organizations already revolutionizing healthcare through secure, intelligent data collaboration.

