Recruitment Reinvented: Partnering with Leading Clinical Trial Companies

The Billion-Dollar Bottleneck in Clinical Research
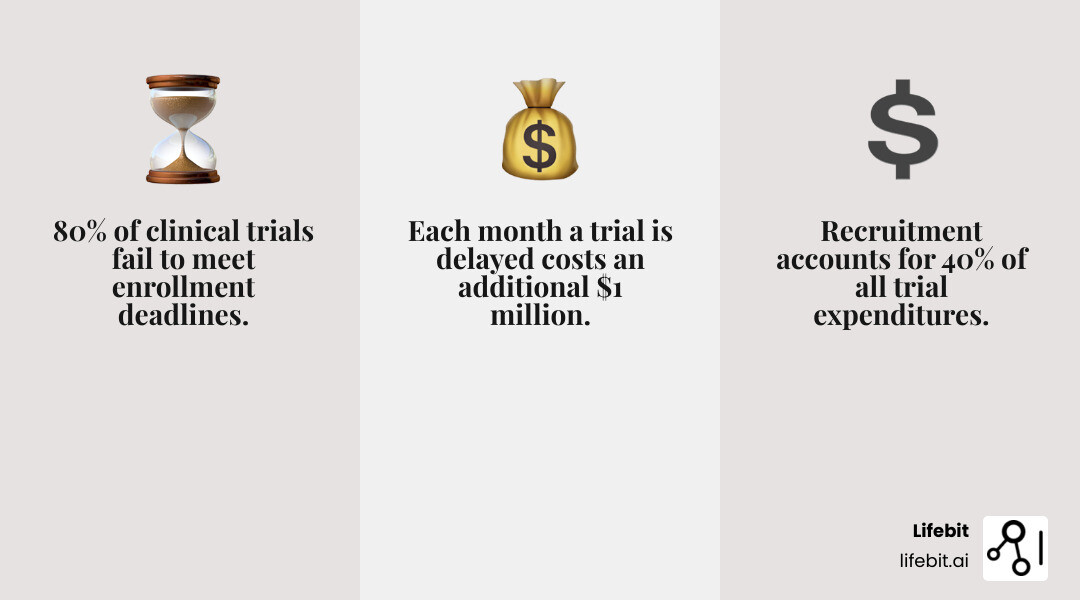
Patient recruitment companies are specialized firms that help sponsors and CROs accelerate clinical trial enrollment through strategies like digital advertising, EHR matching, patient databases, advocacy partnerships, and decentralized trial platforms. These companies address the industry’s most persistent challenge: 80% of clinical trials fail to meet their enrollment deadlines, and recruitment costs account for 40% of all trial expenditures.
Top Patient Recruitment Companies by Capability:
| Company Type | Key Strengths | Best For |
|---|---|---|
| Full-Service Agencies | End-to-end recruitment, site support, retention | Complex multi-site trials |
| Digital-First Platforms | Social media, search ads, mobile engagement | Broad patient outreach |
| Data-Driven Specialists | EHR matching, real-world data, AI cohort identification | Precision targeting |
| Decentralized Trial Experts | Virtual visits, at-home care, telehealth integration | Geographic expansion |
| Rare Disease Focused | Patient registries, advocacy networks, niche populations | Low-prevalence conditions |
The stakes couldn’t be higher. Every month a trial is delayed can cost an additional $1 million, and nearly 85% of clinical trials struggle to recruit enough patients—some fail to enroll a single participant. These delays ripple across the entire drug development lifecycle, pushing back regulatory submissions, delaying patient access to new therapies, and draining budgets that could fund innovation.
The root causes are well-documented: rising protocol complexity, strict inclusion criteria, patient burden, geographic barriers, lack of awareness, and persistent gaps in diversity and representation. Traditional recruitment methods—physician referrals, site-based outreach, print advertising—no longer scale in today’s complex trial landscape. That’s where modern patient recruitment companies step in, leveraging technology, data, and patient-centric strategies to bridge the gap between sponsors and the patients who need them most.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we power federated data platforms that enable pharma and public sector partners to identify patient cohorts faster and more securely than ever before. With over 15 years in computational biology, AI, and genomics, I’ve seen how patient recruitment companies and data-driven platforms can transform trial timelines when they work together to open up access to siloed, real-world patient data.
Related content about patient recruitment companies:
Why 8 out of 10 Clinical Trials Fail to Meet Enrollment Deadlines
The grim statistics paint a clear picture: a staggering 80% of studies fail to meet their enrollment deadlines, and a third of all delays for Phase III studies are directly attributed to difficulties finding patients. This isn’t just bad luck; it’s a symptom of systemic challenges that have intensified as clinical research becomes more sophisticated.
Let’s unpack the core reasons why patient recruitment remains such a persistent bottleneck:
- Protocol Complexity: Modern clinical trials are often designed with increasingly intricate protocols. They demand patients with highly specific inclusion and exclusion criteria, often requiring particular genetic markers, disease stages, or treatment histories. This narrows the eligible patient pool significantly. As one source notes, “clinical trials have become more complex, often requiring participants to have specific lab values or markers.”
- Strict Inclusion/Exclusion Criteria: While essential for scientific rigor and patient safety, overly strict criteria can make finding eligible participants akin to searching for a needle in a haystack. This is particularly true for rare diseases or precision medicine trials where the target population is inherently small.
- Lack of Patient Awareness: Many potential participants simply aren’t aware that clinical trials exist as a care option, or they don’t know how to find information about them. Misconceptions about trials, fear of the unknown, or a lack of trust can also deter participation.
- Geographic Barriers: For traditional, site-based trials, patients must live close enough to a research site to attend frequent appointments. This immediately excludes vast populations, especially those in rural areas or those with limited mobility. This is a significant hurdle, particularly in countries with large geographical expanses like the USA or Canada.
- Site and Patient Burden: Participating in a clinical trial can be demanding. Patients may need to take time off work, arrange childcare, cover travel costs, and undergo numerous tests and procedures. This “time toxicity” and “financial toxicity” are significant deterrents. Patients can incur substantial out-of-pocket costs for travel, accommodation, and missed work. When combined with the logistical challenges of frequent site visits—which can average over 10 visits for a typical Phase III trial—the cumulative burden becomes unsustainable for many, contributing to dropout rates that can exceed 30%. For sites, managing complex protocols, patient schedules, and data collection adds significant operational burden.
- Competition from Other Trials: In active therapeutic areas, multiple trials might be recruiting for similar patient populations simultaneously. This creates a competitive landscape where patients can choose from several options, further fragmenting the already limited pool.
- Poor Diversity and Inclusion: Historically, clinical trials have not adequately represented the full diversity of patient populations. This not only makes recruitment harder but also raises serious ethical concerns about health equity. For example, according to the FDA, in 2020, 75% of trial participants were White, while Black patients made up only 8% and Hispanic patients 11%, despite representing 13.6% and 18.7% of the U.S. population, respectively. This disparity is not just a social issue; it has direct scientific consequences. Genetic variations across ethnic groups can affect a drug’s safety and efficacy, meaning a treatment proven in a homogenous population may not work as well, or could be harmful, in others. If trial populations don’t reflect real-world demographics, the generalizability and equitable impact of new treatments are compromised. As a position statement from Velocity highlighted, “To bring equitable products to market, clinical trials need proportionate representation.” We are heavily invested in ensuring that our solutions help our partners leverage the power of local influence to drive this crucial change.
The financial repercussions of these challenges are staggering. As we mentioned, recruitment costs can make up 40% of all trial expenditures, and every month a trial is delayed can add an additional $1 million to the sponsor’s bill. Indeed, “ninety percent of trials require the original timeline to be doubled to meet enrollment goals.” This unsustainable model underscores the urgent need for innovative solutions, which is where specialized patient recruitment companies truly shine. To understand the full financial impact, we encourage you to read more about the true costs of a delayed clinical trial.
The Modern Playbook: How Top Firms Overcome Recruitment Problems
In response to the escalating challenges, leading patient recruitment companies have evolved their strategies, embracing technology, data, and a patient-first approach. They’re not just finding patients; they’re reinventing how patients engage with clinical research.
Data-Driven Strategies to Find the Right Patients Faster
At the heart of modern recruitment lies data. We’re talking about leveraging vast datasets to identify, target, and engage potential participants with unprecedented precision.
- Real-World Data (RWD) and Electronic Health Record (EHR) Analysis: This is a game-changer. Instead of relying on broad advertising, patient recruitment companies can analyze anonymized RWD and EHRs to pinpoint individuals who meet specific trial criteria. This includes data points like diagnoses, medication history, lab results, and even genetic information. By accessing global data networks that span millions of patient lives across continents like Europe and Canada, companies can identify specific patient cohorts on a massive scale. Our own federated AI platform at Lifebit excels in securely accessing and analyzing such global biomedical and multi-omic data, enabling our partners to find the right patients faster.
- Predictive Analytics and AI-powered Cohort Identification: Advanced algorithms can predict which patient populations are most likely to respond to recruitment efforts and identify optimal recruitment channels. AI can sift through massive datasets to identify potential candidates based on complex inclusion/exclusion criteria, significantly reducing screen failure rates.
- Multi-omic Data Integration: For precision medicine trials, integrating genomic, proteomic, and other ‘omic’ data with clinical information allows for highly targeted recruitment. This is particularly relevant for our work at Lifebit, where our platform is designed to handle and analyze multi-omic data, providing a deeper understanding of patient eligibility.
- Digital Outreach: The digital landscape is a fertile ground for recruitment. Patient recruitment companies use sophisticated digital marketing techniques, including:
- Social Media Targeting: Platforms like Facebook, Instagram, and even TikTok (as seen with Rare Patient Voice’s presence) allow for highly granular targeting based on demographics, interests, and health-related behaviors. Companies like StudyKik leverage social media and mobile technology to engage patients.
- Search Engine Marketing (SEM): Patients often search for information about their conditions or potential treatments online. Effective SEM campaigns can connect them with relevant clinical trial opportunities. AutoCruitment, for example, uses over 1,500 digital channels to connect with patients searching for medical information online.
- Online Patient Communities: Platforms like Carenity, supporting 500,000 patients and caregivers across 1,200 chronic and rare diseases, offer a ready-made audience for targeted outreach. Clariness’ ClinLife Patient Portal receives 15 million views a year, demonstrating the reach of digital patient platforms.
The Rise of Decentralized and Hybrid Trial Models

One of the most impactful shifts in patient recruitment has been the move towards decentralized clinical trials (DCTs) and hybrid models. These approaches bring the trial to the patient, rather than requiring the patient to travel to a site.
- Decentralized Clinical Trials (DCTs): These trials leverage technology to allow patients to participate from their homes or local healthcare providers. This significantly reduces the geographic barriers and patient burden often associated with traditional trials. Science 37, for instance, is a decentralized clinical trial platform that enables remote participation and site-less trials, even operating as the “First & Only FDA-Inspected Direct-to-Patient Site.” They have supported over 185 trials across various therapeutic areas, demonstrating the effectiveness of this model.
- Virtual Participation: Telehealth appointments, remote monitoring, and eConsent processes mean fewer in-person visits. This is particularly appealing for patients with chronic conditions, mobility issues, or those living far from major research centers.
- At-Home Study Visits: For procedures requiring physical presence, mobile nurses or home health professionals can conduct visits at the patient’s residence. Science 37, for example, employs over 150 research-grade nurses to deliver clinical assessments and procedures directly to patients’ homes.
- Wearable Technology Integration: Continuous data collection from wearables and other remote devices provides rich, real-time insights without requiring constant clinic visits, further reducing patient burden.
- Reduced Travel Burden: By minimizing the need for patients to travel, DCTs increase accessibility, making trials a viable option for a much broader and more diverse population across the USA, Europe, and other continents.
- Expanded Geographic Reach: DCTs allow recruitment to extend beyond the immediate vicinity of a physical site, enabling the inclusion of patients from diverse regions, including rural areas that would typically be underserved by traditional research. This is crucial for global trials, which many of our partners conduct across 5 continents.
Building Trust Through Patient-Centric Engagement
Beyond the technological advancements, successful recruitment hinges on building genuine trust and rapport with patients.
- Patient Advocacy Partnerships: Collaborating with patient advocacy groups is invaluable. These organizations have established trust within specific patient communities and can act as powerful conduits for information and referrals. Rare Patient Voice, for example, actively partners with advocacy groups and sources patients through in-person events and direct referrals.
- Community Outreach Programs: Engaging directly with local communities helps build awareness and trust, especially when targeting diverse or underserved populations. Innovative Trials emphasizes community engagement and a patient-centric approach in its global strategies, operating in over 70 countries.
- Physician and Site Support: Empowering referring physicians and trial sites with the tools and information they need to educate patients is critical. Companies like Elligo Health Research use an “Elligo Direct” approach to make it simple for physicians and their patients to take part in clinical trials.
- Clear Communication: Providing transparent, easy-to-understand information about trials, including their purpose, risks, benefits, and time commitment, is paramount. This fosters informed consent and patient confidence.
- Health Equity Focus: Actively working to ensure that trial populations reflect the diversity of the disease burden is not just ethical, but also scientifically sound. Velocity, with its integrated site organization across the USA and Europe, emphasizes prioritizing health equity at the site level to achieve proportionate representation in clinical trials.
- Culturally Competent Materials: Recruitment materials and communication strategies must be culturally sensitive and linguistically appropriate to resonate with diverse patient groups.
- Ensuring Proportionate Representation: Proactive strategies to identify and engage underrepresented groups are essential. This includes partnering with community leaders, utilizing diverse recruitment channels, and designing trials with inclusivity in mind from the outset.
How to Select the Right Patient Recruitment Companies for Your Trial
Choosing the right patient recruitment company is a critical decision that can make or break your trial timelines and budget. It’s not a one-size-fits-all endeavor; the best partner for you depends on your specific trial’s needs, therapeutic area, and target population.
Evaluating the Strategies of Patient Recruitment Companies

When assessing potential partners, we encourage our clients to look beyond surface-level promises and dig into the substance of their strategies.
- Therapeutic Area Expertise: Does the company have a proven track record in your specific disease area? Recruitment for oncology trials differs significantly from rare neurological conditions or cardiovascular studies. CSSi, for example, boasts 15 years of experience across 30+ therapeutic areas and 40+ countries, while Innovative Trials has expertise in over 70 countries and across various therapy areas. This specialized knowledge means they understand the patient journey, the relevant medical terminology, and the most effective outreach channels.
- Scalability from Niche to Large-Scale Trials: Can the company handle the scope of your trial? Whether you’re targeting a rare disease population or launching a global Phase III trial, your partner needs to demonstrate the ability to scale from small, niche studies to large-volume recruitment.
- Technology Platforms: What technological infrastructure do they leverage? Look for companies that use advanced platforms for patient identification, screening, engagement, and data management. This could include AI-powered platforms that match patients to trials based on their health data, patient portals, and mobile apps. For instance, StudyKik uses social media and mobile technology to engage patients.
- Data Privacy and Compliance (GDPR, HIPAA): Given the sensitive nature of health data, robust data privacy and compliance protocols are non-negotiable. For global trials, especially those involving patients in Europe, adherence to GDPR is paramount. Our own Lifebit platform is built with federated governance and secure Trusted Research Environments (TREs) that ensure GDPR+ compliance, allowing for compliant research across hybrid data ecosystems. Any partner you choose must demonstrate the same commitment to data security and ethical handling of patient information.
- Global vs. Local Capabilities: Does the company have the reach and cultural understanding to recruit effectively in all your target geographies? Some companies specialize locally (e.g., within the USA or UK), while others, like MMG, CSSi, Clariness, and Innovative Trials, position themselves as global recruitment strategists with extensive international experience. If your trial spans multiple continents, a partner with proven global capabilities is essential.
- Communication and Reporting Standards: How transparent are they about their progress? A good partner provides clear, regular reporting on recruitment metrics, insights into campaign performance, and proactive communication about any challenges or opportunities.
Key Questions to Ask Potential Patient Recruitment Companies
To ensure you find the perfect match, arm yourself with a set of probing questions:
- Trial Experience: “Can you share case studies or examples of successful recruitment in my therapeutic area and trial phase?” “What is your experience with international trials, particularly in regions like Europe, the USA, or Canada?” (CSSi, for example, has worked in 40+ countries).
- Recruitment Methodologies: “What specific recruitment channels do you plan to use for our trial (e.g., digital ads, EHR matching, advocacy groups, community events)?” “How do you tailor your approach to different patient demographics and conditions?”
- Patient Screening Process: “How will potential participants be screened to ensure eligibility and reduce screen failures?” “Do you offer custom prescreeners, and how do you handle ineligible patients who might be suitable for other trials?”
- Diversity and Inclusion Strategy: “What specific strategies do you employ to ensure a diverse and representative patient population?” “How do you measure and report on participant diversity metrics?” This is a critical area, and a strong answer here reflects a forward-thinking partner. Velocity highlights the importance of proportionate representation for equitable products.
- Performance Metrics: “What key performance indicators (KPIs) do you use to measure success, and how do they align with our trial goals?” “Can you provide examples of how you track cost per enrolled patient, time to enroll, and conversion rates?”
- Budget Models: “What are your typical budget models (e.g., fixed fee, risk-based, performance-based)?” “How can we optimize costs while ensuring effective recruitment?”
- Patient Retention Services: “Beyond initial recruitment, what services do you offer to support patient retention throughout the trial?” “How do you manage patient engagement and follow-up to minimize drop-out rates?” Many companies, like Elligo Health Research, offer customized services for both enrollment and engagement.
Measuring What Matters: KPIs and Cost Optimization for Recruitment
In the high-stakes world of clinical trials, effective patient recruitment isn’t just about filling quotas; it’s about strategic investment. Knowing how to measure success and optimize costs is crucial for maximizing your return on investment and accelerating drug development.
The Essential Metrics That Define Recruitment Success
We can’t improve what we don’t measure. For patient recruitment companies, a robust set of Key Performance Indicators (KPIs) provides invaluable insights into campaign effectiveness and overall trial progress.
- Cost Per Enrolled Patient (CPEP): This is perhaps the most critical financial metric. It tells you how much it costs to successfully enroll one qualified patient into your trial. By tracking CPEP across different recruitment channels and partners, you can identify the most cost-effective strategies.
- Time to First and Last Patient In: Speed is money in clinical trials. Tracking how long it takes to enroll the first patient and, crucially, to reach full enrollment helps assess efficiency and predict timelines. “Every month delayed can cost an additional $1 million.”
- Channel-Specific Conversion Rates: How many website visitors become pre-screened? How many pre-screened patients qualify for a full screening? How many screened patients are randomized? Analyzing conversion rates at each stage of the funnel for different channels (e.g., social media ads, physician referrals, patient advocacy groups) reveals where your efforts are most effective and where bottlenecks occur.
- Screen Failure Rate: A high screen failure rate indicates that your recruitment efforts are not reaching the right patients, or your screening process is inefficient. Good patient recruitment companies aim to minimize this by pre-qualifying patients rigorously.
- Participant Diversity Metrics: Beyond sheer numbers, measure the diversity of your enrolled population against relevant demographic benchmarks. This includes age, gender, ethnicity, and geographic location, ensuring your trial is representative and promotes health equity.
- Patient Retention Rate: Recruitment is only half the battle. A high drop-out rate negates initial recruitment success. Monitoring retention rates provides insight into patient engagement and satisfaction throughout the trial.
Strategies to Maximize Your Recruitment ROI
Optimizing your recruitment investment requires a strategic approach that combines data analysis, flexible partnerships, and a focus on patient experience.
| Recruitment Channel | Cost-Effectiveness | Reach Potential | Engagement Level | Time to Enroll |
|---|---|---|---|---|
| Digital Ads | Medium to High | High | Medium | Fast |
| EHR Matching | High | Medium | High | Fast |
| Advocacy Groups | High | Niche | Very High | Medium |
| Physician Referrals | Medium to High | Medium | High | Medium |
| Community Events | Medium | Local | High | Slow to Medium |
| Patient Databases | Medium | High | Medium | Fast |
- Optimizing Channel Mix: Don’t put all your eggs in one basket. A diversified strategy, leveraging a mix of digital, data-driven, and community-based channels, often yields the best results. For instance, while digital ads offer broad reach, EHR matching provides precision, and advocacy groups offer deep trust within specific communities. AutoCruitment uses over 1,500 digital channels, while Rare Patient Voice relies on advocacy partnerships and in-person events.
- Performance-Based Contracts: Consider contracting with patient recruitment companies using models tied to specific performance metrics. These models can vary. A “pay-per-enrolled-patient” model is common, but more sophisticated agreements might include milestone payments for hitting enrollment velocity targets or bonuses for achieving diversity quotas. Some sponsors opt for a “risk-sharing” model, where the recruitment firm covers some initial outreach costs for a higher payout upon success. This contrasts with traditional “fee-for-service” models based on activities rather than outcomes. Choosing the right model aligns incentives and requires a clear understanding of the trial’s difficulty and the sponsor’s risk tolerance.
- Reducing Patient Drop-out: Proactive patient engagement and support services are key. This includes clear communication, concierge services, travel support, and using decentralized trial models to reduce burden.
- Investing in Retention: A patient-centric approach from day one, coupled with continuous engagement, can significantly improve retention. Companies like BBK Worldwide offer patient-centric solutions and concierge support, recognizing that retention starts with recruitment.
By carefully tracking these metrics and strategically optimizing your approach, we can move away from the “trial and error” of traditional recruitment and towards a more predictable, cost-effective, and patient-friendly process.
The Future: AI, Federated Data, and the End of Recruitment Delays
The future of clinical trial recruitment is being built on artificial intelligence and vast new sources of health data. This shift promises to eliminate recruitment delays by replacing slow, manual processes with precision, efficiency, and better patient access.
- Predictive Analytics for Enrollment Forecasting: Imagine knowing with high accuracy how long it will take to enroll your trial and which recruitment channels will be most effective. Advanced AI and machine learning are making this a reality. By analyzing historical data from thousands of trials, these systems can forecast enrollment trends, identify potential roadblocks, and recommend proactive adjustments, saving millions in potential delays.
- Real-time Evidence Generation: The ability to generate real-time evidence from diverse patient populations, both for recruitment and for pharmacovigilance, is becoming paramount. This allows for continuous learning and adaptation throughout the trial lifecycle, leading to more robust and relevant research outcomes.
- The Shift to Patient-Centric Trial Design: Technology and data are enabling a profound shift towards designing trials around the patient. This means considering their preferences, reducing their burden through decentralized models, and ensuring their voices are heard throughout the research process. This patient-first approach is not just ethical; it’s a powerful driver of recruitment and retention.
- Federated AI Platforms for Secure Data Access: The key to this change is the ability to securely access and analyze vast, disparate datasets without compromising patient privacy. This is where federated AI platforms come into play. They allow researchers to query data across multiple institutions (e.g., hospitals, biobanks, research centers in the USA, UK, Europe, Canada, and beyond) without the data ever leaving its source. This means access to billions of patient data points, including complex multi-omic data, for cohort identification, while maintaining strict data governance and privacy standards like GDPR+.
- Lifebit’s Role in Powering Next-Generation Research: At Lifebit, we are at the forefront of this change. Our next-generation federated AI platform enables secure, real-time access to global biomedical and multi-omic data. With built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance, our platform powers large-scale, compliant research and pharmacovigilance across biopharma, governments, and public health agencies. Components like our Trusted Research Environment (TRE) and Real-time Evidence & Analytics Layer (R.E.A.L.) deliver real-time insights and AI-driven safety surveillance. This allows patient recruitment companies and our partners to leverage previously inaccessible data to precisely identify eligible patients, accelerate enrollment, and ensure trials are representative of the real world. By integrating this intelligence, sponsors and their recruitment partners can make recruitment delays the exception, not the rule.
By combining the expertise of leading patient recruitment companies with cutting-edge federated data platforms, the industry can bring new therapies to patients faster. Explore how Lifebit’s platform can directly impact your recruitment timelines and trial success. Explore Lifebit’s federated data platform.

