The Future of Clinical Research Industry Trends You Cannot Ignore

Future Trends in Clinical Research: Cut Trial Timelines by 30% with AI
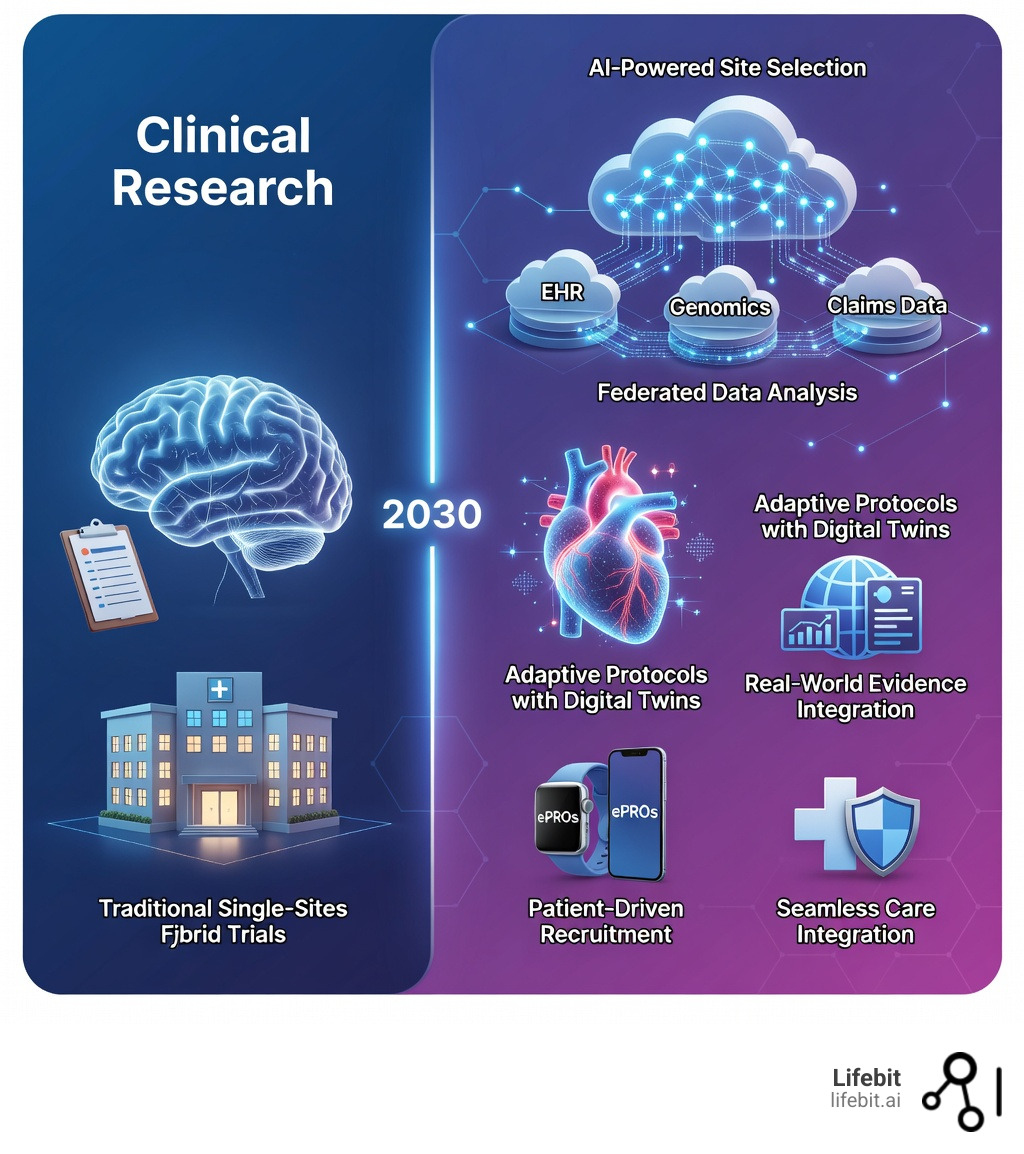
Future trends in clinical research are no longer distant possibilities—they’re here, reshaping how trials run, who can participate, and how quickly therapies reach patients. The clinical research industry is entering a defining era where scientific innovation, operational complexity, and rising expectations for speed and quality are converging. Here’s what you need to know:
Key Trends Changing Clinical Research:
- AI and Machine Learning – Reducing trial timelines by up to 30% and costs by 20% through predictive modeling, automated protocol generation, and risk-based monitoring
- Decentralized and Hybrid Trials – Now comprising 40% of new trials, expanding access for diverse populations through remote monitoring and digital tools
- Real-World Evidence (RWE) – FDA-accepted data from electronic health records and claims supplementing traditional trials with long-term, diverse population insights
- Precision Medicine – Multi-omics sequencing and biomarker-driven designs increasing treatment response rates from 20% to 42%
- Regulatory Innovation – Single IRB models and continuous trial frameworks accelerating approval timelines and reducing redundancy
- Patient-Centric Design – Digital tools, wearables, and patient-reported outcomes driving 30% higher retention rates in inclusive trial designs
- Advanced Therapies – Cell, gene, and CRISPR therapies requiring specialized site readiness and novel endpoint strategies
The COVID-19 pandemic accelerated what was already underway: a fundamental shift from hospital-centric, one-size-fits-all trials to technology-enabled, patient-driven research. Phase III trials have increased two-and-a-half times since 2000, yet 60-80% of patients with conditions like heart failure and cystic fibrosis still cannot access trials where they receive care. Meanwhile, AI can now prepare essential trial documents up to 50% quicker, and minority representation in trials increased by 25% from 2018 to 2022.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve spent over 15 years building federated data platforms that power secure, compliant clinical research across siloed datasets. Understanding future trends in clinical research means recognizing that success now depends on connecting diverse data sources, embedding AI into workflows, and putting patients at the center of every decision.
Essential future trends in clinical research terms:
- AI-Powered Clinical Trials: Real-World Examples Transforming Research in 2025
- Advanced analytics healthcare
AI in Clinical Research: Cut Timelines, Cut Costs
If you feel like the traditional clinical trial model is too slow and too expensive, you aren’t alone. The industry is currently moving away from the “pilot” phase of Artificial Intelligence and into full-scale production. Research suggests that AI-powered protocol automation is no longer a “nice-to-have” but a critical operational reality for 2026. AI and Machine Learning (ML) are tackling the “white space” in clinical development—those long periods of administrative delay that add months to a drug’s time-to-market.

The Shift from Pilot to Production
By using AI to scan historical data, sponsors can now predict which sites will actually meet enrollment targets and which will fail before the first patient is even screened. This isn’t just about speed; it’s about survival in a market where Phase I to approval success rates still hover below 10%. We are seeing the rise of Generative AI (GenAI) in drafting complex documents. Instead of medical writers spending weeks on a first draft, LLMs trained on regulatory-compliant datasets can produce high-quality protocols in hours, which are then refined by human experts. This “human-in-the-loop” approach ensures that the speed of AI is balanced with the clinical judgment of seasoned researchers.
How Lifebit’s AI Transforms Clinical Research Operations
At Lifebit, we see the future of clinical operations as a “human-in-the-loop” automated ecosystem. Our platform enables:
- Automated Document Generation: AI can now draft protocols, informed consent forms, and clinical study reports up to 50% faster with fewer manual errors. This includes the automated mapping of data to CDISC standards, which traditionally consumes thousands of man-hours.
- Smart Site Selection: Instead of relying on gut feelings or outdated databases, we use predictive analytics to identify high-performing sites based on real-world patient density, historical enrollment speed, and past data quality scores.
- Risk-Based Validation and Monitoring (RBM): Regulators are increasingly open to machine-generated evidence for validation. AI algorithms can now monitor trial data in real-time, flagging outliers or potential fraud immediately rather than waiting for a quarterly monitoring visit. This reduces the manual testing burden, allowing teams to focus on high-risk areas while maintaining strict compliance.
- End-to-End Automation: From site activation to data cleaning, AI agents are beginning to handle repetitive workflows. For instance, AI-driven data cleaning can automatically query inconsistent entries, reducing the time from “Last Patient, Last Visit” to database lock by weeks.
AI-Driven Decisions and Talent for Faster Trials
The future trends in clinical research aren’t just about the tech; they’re about the people using it. We are seeing a shift toward an upskilled, customer-centric talent model. Clinical Research Associates (CRAs) are evolving into data scientists and relationship managers who use AI-driven decision engines to make real-time adjustments to trial conduct. This “fit-for-portfolio” approach means that a trial for a rare disease (where every single patient is a miracle) is managed differently than a large-scale chronic disease trial. By 2035, we expect AI to autonomously manage site activation and patient identification, making trials a seamless part of the standard care experience.
Decentralized Trials: End Recruitment Bottlenecks
Recruitment is the single biggest bottleneck in clinical research, responsible for nearly 80% of trial delays. Did you know that only 10% of people with schizophrenia in the U.S. can participate in a trial at the clinic where they already receive care? For many, the “commute to a cure” is over an hour long, which is a massive barrier to participation, particularly for those in rural or underserved communities.
Decentralized Clinical Trials (DCTs) are the solution. As of 2024, roughly 40% of new trials incorporate decentralized elements, such as mobile nursing, local lab visits, or home-based monitoring. This shift is not just about convenience; it is about democratizing access to life-saving medicine.
| Feature | Traditional Trials | Decentralized/Hybrid Trials |
|---|---|---|
| Patient Location | Must travel to academic centers | Participates from home or local clinics |
| Data Collection | Manual entry at site visits | Real-time via wearables and ePROs |
| Recruitment | Limited to site geography | Global reach via digital platforms |
| Retention | High burden, higher dropout | Lower burden, 30% higher retention |
Boosting Patient Engagement with Lifebit’s Digital Tools
To keep patients engaged, we have to meet them where they are—on their phones and in their daily lives. Lifebit’s ecosystem supports the integration of digital health tools that transform the participant experience:
- ePROs (Electronic Patient-Reported Outcomes): Instead of trying to remember how they felt three weeks ago, patients report symptoms in real-time. In a migraine study, for example, reporting severity immediately leads to much higher data accuracy and reduces recall bias.
- Wearables and IoMT: Smartwatches and medical-grade sensors provide continuous data on heart rate, sleep, and activity levels. This “Internet of Medical Things” (IoMT) allows for the collection of digital biomarkers that were previously impossible to capture in a clinic setting.
- Virtual Visits and Telemedicine: Telemedicine check-ins remove the logistical nightmare of travel, especially for elderly or disabled participants. This is supported by eConsent platforms that allow patients to review and sign documents from their own living rooms.
- Gamification and Support: We use interactive platforms and milestones to keep patients motivated. For long-term studies, providing patients with insights into their own data (where ethically permitted) can significantly boost long-term adherence.
Diversity, Equity, and Inclusion (DEI) in Modern Trials
Diversity is no longer just a “good idea”—it’s a regulatory mandate. The FDA’s Diversity Action Plans (DAPs) now require sponsors to proactively include underrepresented groups. Inclusive designs aren’t just about ethics; they lead to 30% higher retention rates and ensure that the trial results are generalizable to the actual patient population. By breaking geographic barriers through DCTs, we can reach minority populations who have historically been excluded from research. Lifebit’s federated platform allows us to tap into diverse datasets across 5 continents, ensuring that the therapies of tomorrow work for everyone, not just a narrow demographic. This includes addressing the “Digital Divide” by providing participants with the necessary hardware and data plans to participate in tech-enabled trials.
Real-World Evidence and Multi-Omics: Precision at Scale
The “gold standard” Randomized Controlled Trial (RCT) is being joined by a powerful partner: Real-World Evidence (RWE). By looking at data from electronic health records (EHRs), insurance claims, and even pharmacy records, we can see how a drug performs in the “wild.” This is particularly crucial for identifying rare side effects or long-term benefits that a 6-month trial might miss.
Synthetic Control Arms and Data Integration
One of the most exciting future trends in clinical research is the use of Synthetic Control Arms (SCA). By using RWE from historical trials and EHRs, researchers can create a “virtual” placebo group. This is a game-changer for rare disease trials where it is ethically difficult or logistically impossible to recruit a large control group. Lifebit’s platform facilitates this by securely connecting disparate data sources, allowing for the creation of robust, regulatory-grade synthetic cohorts.
Patient-Centric Trials and Anti-Obesity Study Problems
Anti-obesity trials (GLP-1s) are currently exploding, with over 120 molecules in the pipeline. However, these trials face unique challenges like “motivational fatigue.” Patients often drop out when they hit a weight-loss plateau or if the side effects (like nausea) become too much to handle. Future trends for obesity research focus on:
- Behavioral Insights: Using AI to spot patterns of disengagement early—such as a patient missing their daily log entries—and providing personalized lifestyle support from dietitians.
- Transparent Communication: Setting realistic expectations about plateaus via digital apps to prevent frustration.
- Functional Outcomes: Measuring not just weight loss, but “quality of life” metrics—can the patient walk their dog or play with their kids? These patient-centric endpoints are becoming as important as the scale.
Advanced Therapies: From Cell Therapy to CRISPR
We are entering the age of “cures” rather than just “treatments.” Cell and gene therapies (CGTs) and CRISPR-based edits are reaching patients, but they are operationally complex. These trials require specialized site readiness, as sites must be equipped to handle complex logistics like cryopreservation and long-term follow-up (sometimes up to 15 years). We are seeing the rise of “Hub-and-Spoke” models, where smaller community sites partner with “hubs” of expertise to allow more patients access to these life-altering therapies. Furthermore, the integration of multi-omic data (genomics, proteomics, and transcriptomics) allows for unprecedented patient stratification, ensuring the right patient gets the right gene edit at the right time. With Lifebit’s Trusted Research Environment (TRE), researchers can analyze this sensitive data without it ever leaving its secure home, maintaining the highest standards of patient privacy.
Regulatory Innovation and Site Readiness for 2026
Regulatory bodies like the FDA and MHRA are moving faster than ever, adopting a more flexible, risk-based approach to oversight. One of the biggest shifts is the Single IRB (Institutional Review Board) model, which will soon be mandatory for multi-site trials. This eliminates the need for every single hospital to do its own ethical review, cutting months off the study start-up timeline and reducing the administrative burden on site staff.
Quality by Design (QbD) and ICH E6(R3)
The upcoming ICH E6(R3) guidelines emphasize “Quality by Design.” This means building quality into the protocol from the beginning, rather than trying to “inspect it in” at the end. By identifying the “factors critical to quality” early on, sponsors can avoid the 15-20% of protocol amendments that are currently caused by avoidable design flaws. This proactive approach is essential for the complex, multi-arm trials that are becoming the industry standard.
Lifebit-Powered Site Partnerships and Ecosystem Growth
The relationship between sites and sponsors is moving from “transactional” to “strategic.” Instead of starting from scratch for every trial, sponsors are building long-term networks with pharmacies, community clinics, and other non-traditional care settings. Lifebit helps scale these ecosystems by providing a “Trusted Data Lakehouse” where sites and sponsors can collaborate in real-time. This ensures that everyone is looking at the same high-quality data, reducing the 78% of delays that are currently caused by poor communication and fragmented data silos.
Adaptive Protocols and Digital Twins for Smarter Trials
Why wait until the end of a trial to realize a dose is too low? Adaptive protocols allow for real-time changes based on emerging data, such as dropping an ineffective treatment arm or increasing the sample size mid-study. This flexibility saves time and resources while ensuring patient safety.
Furthermore, the emergence of Digital Twins—virtual models of a patient’s biology—is set to revolutionize early-phase research. By simulating how a patient might respond to a drug based on their genetic and clinical profile, researchers can de-risk development before a single dose is administered. This is particularly valuable for early-stage biotechs that need to prove efficacy quickly to secure funding. As we move toward 2030, these digital models will become increasingly sophisticated, eventually allowing for “in silico” trials that can supplement or even replace certain phases of human testing.
Frequently Asked Questions about Future Trends in Clinical Research
How does Lifebit’s AI reduce clinical trial costs and timelines?
Our AI-driven platform automates tedious tasks like document generation and site selection, reducing timelines by up to 30%. By using federated learning, we also allow researchers to access global data without the high costs of data moving or duplication.
What are the main benefits of decentralized clinical trials with Lifebit?
Lifebit enables decentralized trials by providing a secure infrastructure for remote data collection. This increases patient access, improves diversity, and boosts retention by allowing participants to engage with the trial from the comfort of their own homes.
How are regulatory changes like single IRB impacting trial start-up?
The shift to a single IRB model streamlines the approval process for multi-site trials. Instead of waiting for dozens of local boards, a single review covers all sites, potentially cutting months off the “white space” between protocol finalization and the first patient in.
Conclusion
The future trends in clinical research are clear: the industry is becoming more digital, more decentralized, and infinitely more data-driven. Success in 2026 and beyond will be defined by those who can bridge the gap between scientific innovation and operational reality.
At Lifebit, we are proud to be at the heart of this change. Our federated AI platform and Trusted Research Environment provide the security, compliance, and real-time insights needed to power the next generation of clinical research. Whether it’s through multi-omic integration or AI-driven safety surveillance, we are helping biopharma and public health agencies deliver life-saving therapies to patients faster than ever before.
Ready to see how we can accelerate your research? Learn more about the Lifebit platform and join the shift toward a more patient-centric future.

