ADR Surveillance
Unlock real-world safety insights with a trusted ADR Surveillance Platform
Securely collect, connect, and analyze post-market and clinical safety data—accelerating signal detection, validation, and regulatory compliance while maintaining full data control.
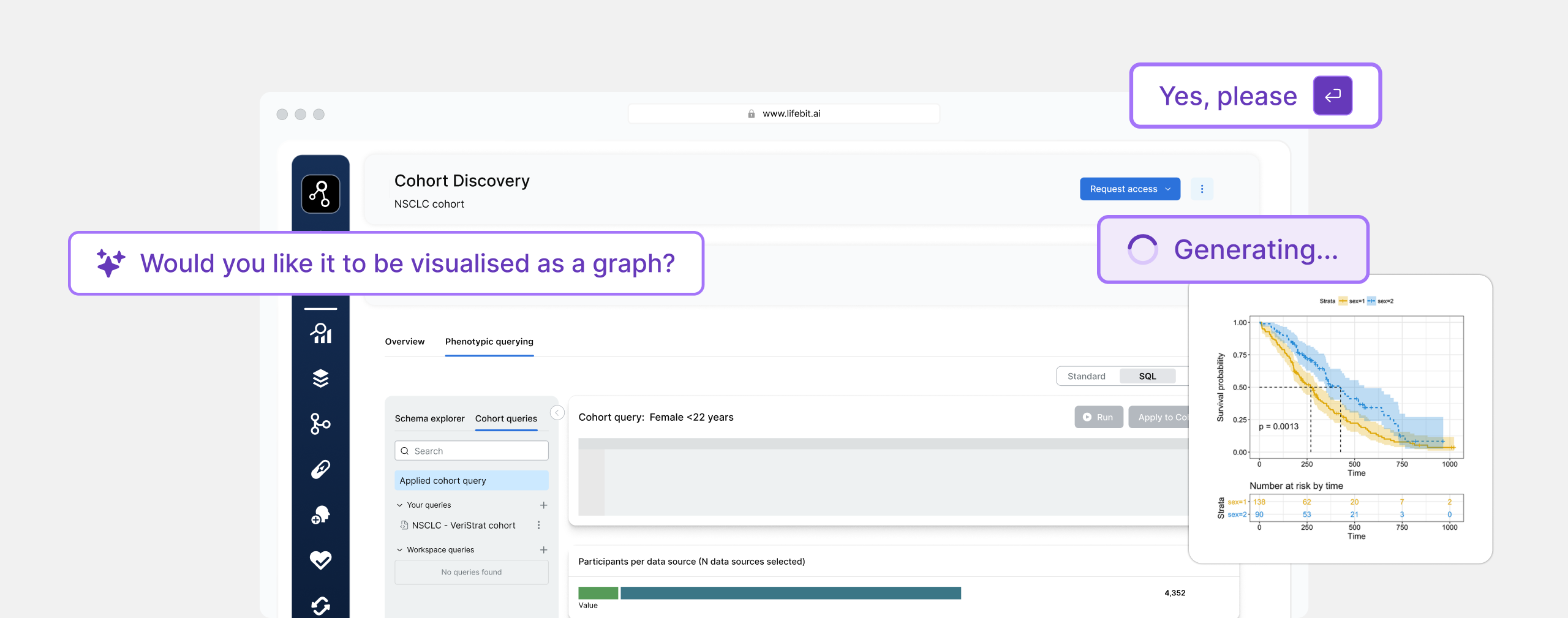
Unlock the power of ADR surveillance, securely
Lifebit’s ADR Surveillance Platform connects regulators, health systems, and sponsors to analyze and collaborate across safety data, without moving it or compromising privacy.
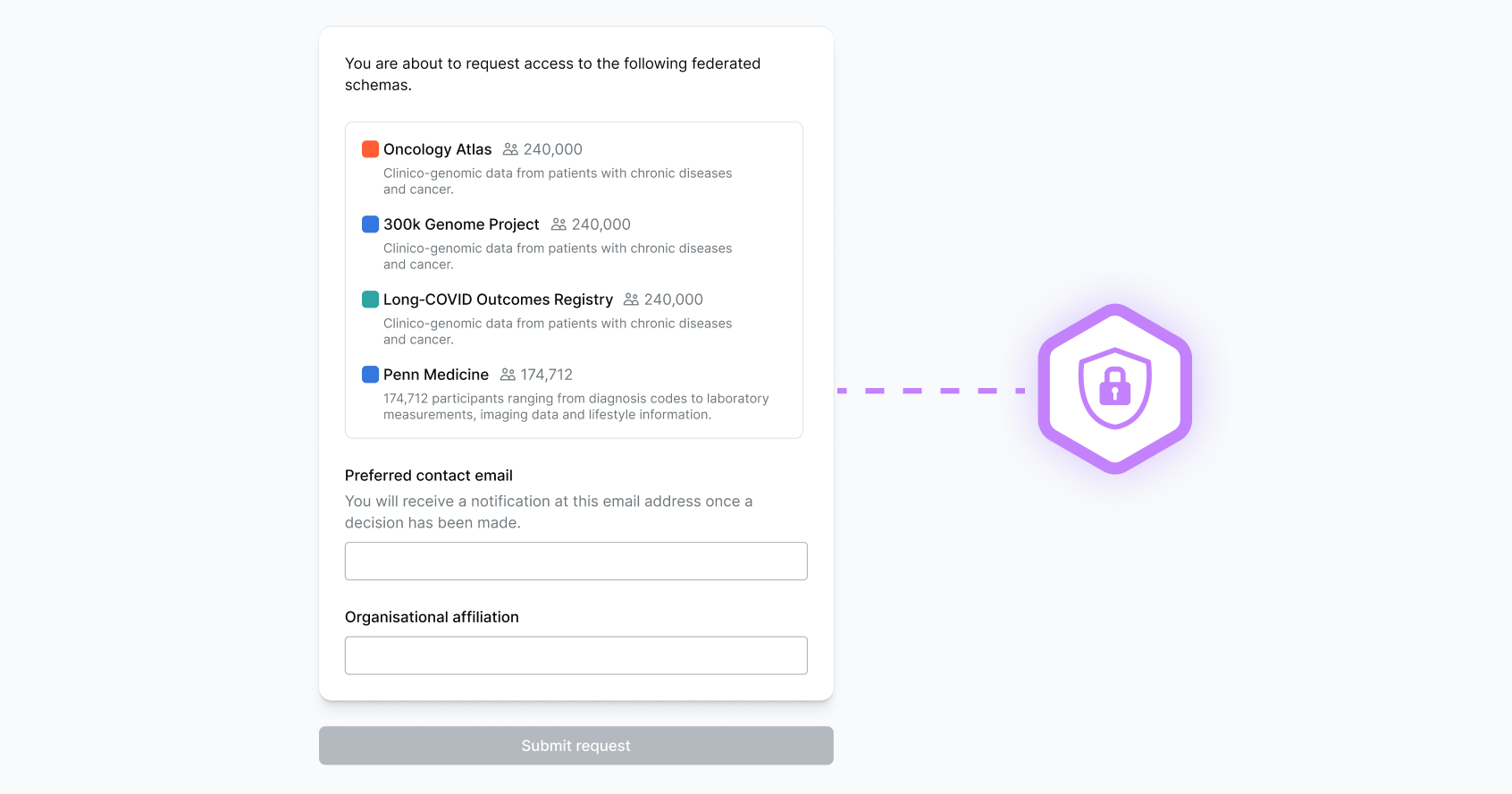
Federated Data Access & Analysis
Connect safety data without transferring sensitive information
Enable regulators, pharma, and research networks to collaborate securely across distributed environments—analyzing ADR data while maintaining local data governance.
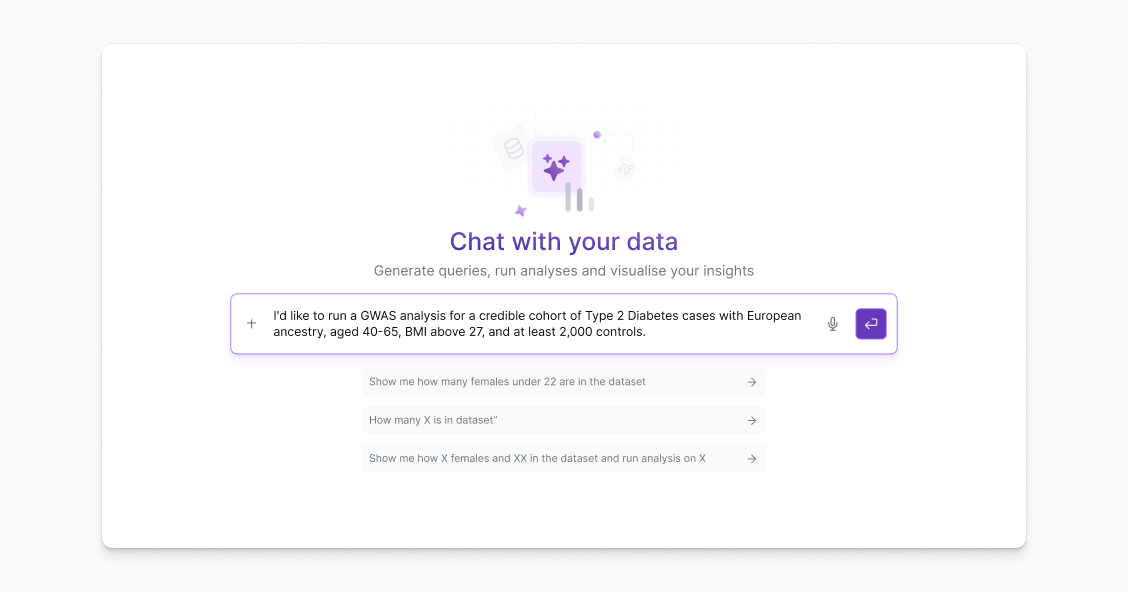
AI-Powered Signal Detection
Enhance pharmacovigilance with Lifebit’s AI engine
Use Lifebit’s AI-driven analytics to detect emerging safety trends across diverse data sources—accelerating signal detection and improving decision-making accuracy.
Compliance & Audit-Ready Reporting
Ensure regulatory compliance with full transparency
Automate reporting and documentation in line with FDA and EMA pharmacovigilance requirements—ensuring data integrity, auditability, and end-to-end traceability.

Work Together
Great work doesn’t happen alone. Let our experts show you how our product suite eliminates silos and unifies strategy.
Resources to power your development