Why Patient Recruitment is Key to Clinical Trial Success

Clinical trial patient recruitment: 3 Essential Strategies for Success
Why Clinical Trial Patient Recruitment Determines Research Success
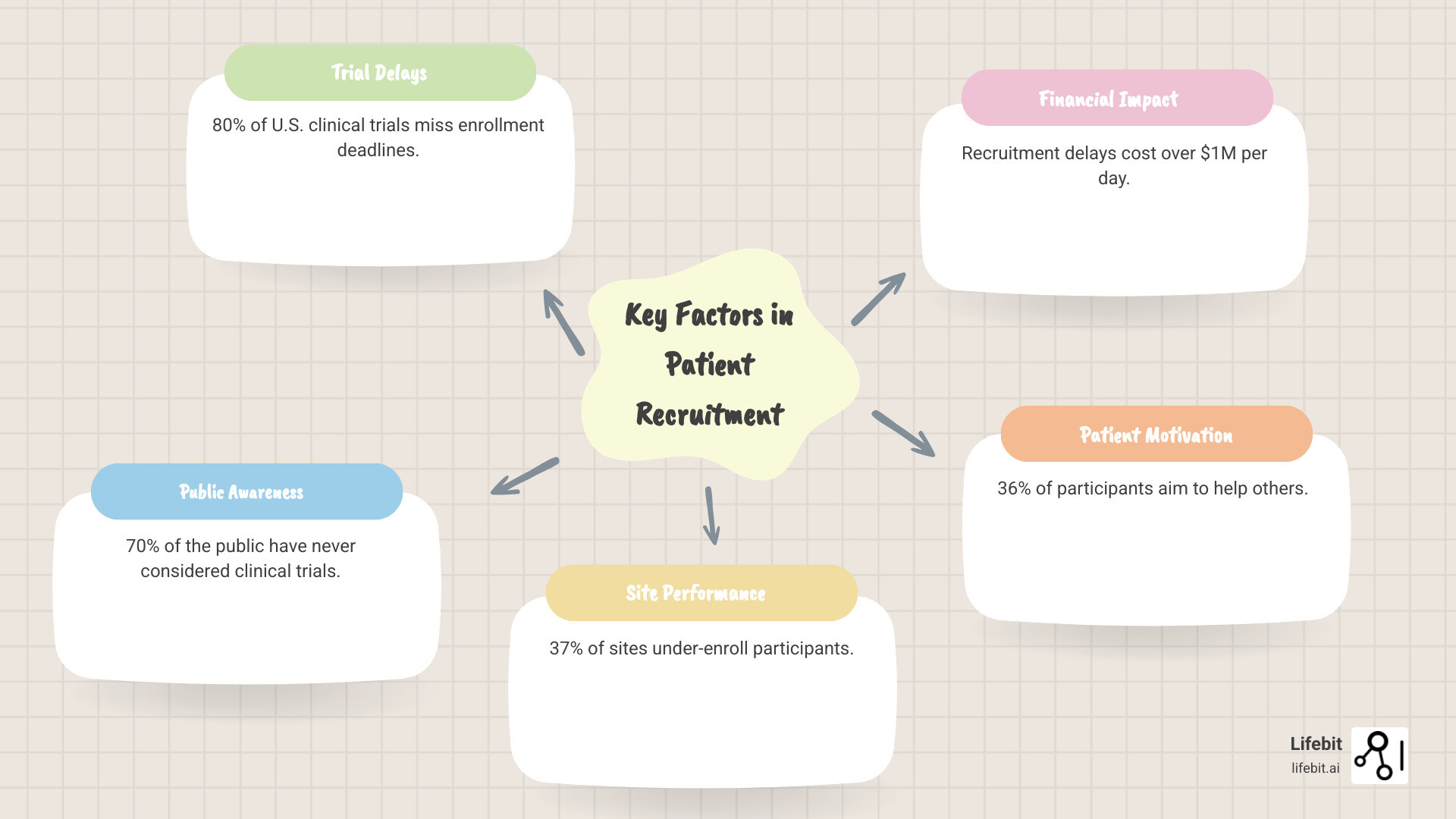
Clinical trial patient recruitment is the foundation that determines whether medical breakthroughs reach patients. Yet with 80% of U.S. clinical trials missing enrollment deadlines, finding the right participants has become the critical bottleneck in medical innovation.
Key factors in clinical trial patient recruitment:
- Primary information sources: 58% learn through physicians, 40% via online registries, 30% through search engines
- Main motivations: 36% want to help others, 26% aim to advance medicine, 15% seek personal health benefits
- Decision factors: 83% consider risks/benefits, 75% evaluate study purpose, 73% assess procedures required
- Current challenges: 37% of sites under-enroll, 11% fail to enroll anyone, 70% of public never considered trials
The stakes are immense. Recruitment delays cost hundreds of thousands to over a million dollars per day and can shut down promising studies. These challenges extend beyond budgets, touching on patient trust, awareness, and whether research serves the diverse populations it aims to help.
For many research teams, recruiting participants poses a bigger challenge than the trial itself, as protocols become more complex and competition for participants intensifies.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where I’ve spent over 15 years changing how we approach clinical trial patient recruitment through federated data analysis and AI-powered matching across global healthcare datasets. My experience in computational biology and health-tech entrepreneurship has shown me how the right technology can turn recruitment from a bottleneck into an accelerator for medical findings.
Glossary for clinical trial patient recruitment:
- clinical trial recruitment strategies
- digital clinical trial recruitment
- clinical trial recruitment digital results
The High Stakes: Common Challenges in Clinical Trial Recruitment
When clinical trial patient recruitment fails, the consequences ripple far beyond missed deadlines. Studies get terminated, promising treatments disappear into regulatory limbo, and patients lose access to potentially life-changing therapies. The financial toll is staggering, with delays costing up to a million dollars per day, jeopardizing not only the specific drug in development but also the financial viability of the sponsoring organization.
The root causes are complex. Protocol complexity has skyrocketed, creating narrow patient pools, while public awareness remains low—70% of people have never even considered clinical trials as a treatment option when talking with their doctors. This gap between scientific need and public engagement creates a perfect storm of scientific ambition meeting real-world limitations. Understanding these challenges is the first step toward building better recruitment strategies. For insights into how innovative approaches are tackling these issues, check out Innovations in Clinical Trial Recruitment and Enrollment.
Key Challenges in Clinical Trial Patient Recruitment
At the ground level, the numbers are sobering: 37% of sites consistently under-enroll volunteers, while 11% fail to enroll anyone at all. This isn’t just a matter of effort; it’s a systemic issue. Strict eligibility criteria, designed to ensure scientific rigor, have become a major bottleneck. For example, a modern oncology trial might require patients to have a specific genetic mutation, fall within a narrow age range, have failed two specific prior therapies, and have no history of certain comorbidities like heart disease. Each criterion exponentially shrinks the pool of eligible participants, making recruitment a search for a needle in a haystack.
Site performance also suffers from competing priorities. Research coordinators are often overworked, juggling multiple studies, extensive data entry, regulatory paperwork, and patient care. Without dedicated recruitment staff or adequate training, recruitment often becomes a secondary task. Practical issues like participant availability and scheduling conflicts add another layer of friction. Patients may face transportation difficulties, an inability to take time off work, or family care commitments, making the frequent site visits required by traditional trials untenable.
A recent study team documented their real-world experience navigating these exact challenges, offering valuable lessons for other researchers facing similar problems: Insights from a new study team’s experience.
The Patient Perspective: Perceived Risks and Barriers
From the patient’s point of view, the decision to participate is fraught with genuine concerns. Fear of side effects tops the list, worrying 40% of potential participants, while 33% worry about overall health risks. Others are concerned about receiving a placebo (7%) or stopping current, effective treatments (7%). These fears are compounded by logistical and financial burdens. The “financial toxicity” of a trial—including costs for travel, accommodation, and lost wages—can be a significant deterrent, even if the study drug is free.
Beyond medical and financial concerns, a broader trust issue exists, often rooted in historical abuses like the Tuskegee Syphilis Study and a persistent lack of clear, accessible information. This contributes to a confidence gap: 40% of people aren’t confident they could find a suitable clinical study, even if they wanted to participate. The information barrier is just as real as the fear barrier. Many people simply don’t understand how clinical trials work, what protections like Institutional Review Boards (IRBs) are in place, or how to evaluate if a study is right for them. This is particularly true for communities with lower health literacy or limited internet access, creating a digital divide that further exacerbates recruitment disparities.
For a comprehensive look at these patient perspectives and the data behind them, this study provides invaluable insights: Data on patient perceptions and insights.
Most of these barriers can be addressed through better communication, patient-centered trial designs, and technology that makes participation more accessible. Understanding these challenges is crucial for developing effective clinical trial patient recruitment strategies.
A Blueprint for Success: Effective Clinical Trial Patient Recruitment Strategies
Successful clinical trial patient recruitment isn’t about a single magic bullet but a comprehensive, patient-centered approach. The best strategies use a multimodal approach, weaving together multiple touchpoints to reach different communities while keeping the patient’s experience at the heart of everything.

Effective recruitment combines community engagement to build trust, site enablement to empower research teams, and patient-centricity that prioritizes convenience and clear communication. Recruitment isn’t something you do to patients—it’s something you do with them. Data shows that in-person recruitment and personal referrals remain highly efficient, but the most effective teams blend these traditional methods with digital strategies. They continuously track enrollment rates, measure source attribution, and gather participant feedback to turn recruitment from guesswork into a science. For more detailed tactics, explore our guide on Clinical Trial Recruitment Strategies.
Understanding Patient Awareness and Motivations
To recruit effectively, you must understand how people learn about trials and what motivates them. Primary care physicians are the top source (58%), but many patients are also proactive, with 40% using online clinical trial registries and 30% using search engines. This highlights the need for a two-pronged strategy: educating healthcare providers while also optimizing for online discovery.
Interestingly, the top motivation is altruism: 36% want to help others, and 26% want to advance medicine. Only 15% are primarily driven by improving their own condition. This suggests that messaging should emphasize the opportunity to contribute to a greater good, framing participation as a heroic act for future generations. When deciding to join, patients are thoughtful: 83% weigh risks and benefits, 75% consider the study’s purpose, and 73% assess the required procedures. This underscores the need for crystal-clear, honest communication that provides plain-language summaries, transparent consent forms, and direct access to research staff to answer questions. For deeper insights into how patients make these decisions, check out this research: Data on patient decision-making factors.
Traditional vs. Digital Recruitment Channels
The key is not choosing between traditional or digital channels but blending them strategically. Traditional channels like physician referrals, flyers in clinics, and community health fairs excel at building deep, personal trust and often have higher conversion rates. However, their geographic reach is limited and they can be slow to scale.
Digital channels offer vast reach and precise targeting. With up to 40% of patients using social media to find trial information, these channels are vital. This includes running geo-targeted ad campaigns on platforms like Facebook and Instagram, engaging with patient communities on forums like PatientsLikeMe or disease-specific subreddits, and using search engine marketing (SEM) to capture individuals actively looking for treatment options. These channels also provide real-time analytics, allowing teams to A/B test messaging, monitor cost-per-lead, and continuously optimize campaigns for better performance.
The winning strategy combines the trust of traditional methods with the reach of digital channels. Use digital outreach for broad awareness and initial interest, then leverage personal interactions—whether through a trusted physician or a dedicated study coordinator—to build the relationship needed for enrollment. For a deep dive into maximizing digital strategies, explore our insights on Digital Clinical Trial Recruitment.
| Category | Traditional Channels | Digital Channels |
|---|---|---|
| Cost | Lower per patient for targeted local efforts; higher for mass media | Variable, can be highly cost-effective with precise targeting |
| Reach | Local, community-based, limited by geography | Global, scalable, vast diverse audiences |
| Targeting | Relies on provider knowledge and community connections | Highly precise demographic and behavioral targeting |
Optimising Site-Based, Centralised, and Decentralised Models
Effective strategies blend different operational models for recruitment. Site-based recruitment has a built-in advantage, as 72% of clinical trial participants are already patients at the enrolling site, where trust is established. Maximizing this requires empowering site teams with easy-to-use tools, clear protocols, and financial incentives for successful enrollment.
Centralized recruitment models cast a wider net through specialized agencies or platforms, delivering highly qualified, pre-screened leads to sites. This reduces the administrative burden on site staff and accelerates enrollment by ensuring that only motivated and likely-eligible candidates are passed along.
A hybrid model is often ideal, but the biggest evolution is the integration of decentralized clinical trial (DCT) elements. DCTs reduce patient burden by using technology to bring the trial to the patient. This can include telehealth visits for consultations, wearable devices for remote data collection, mobile apps for patient-reported outcomes, and direct-to-patient shipment of study medication. By removing the need for frequent travel to a physical site, DCTs make participation more convenient and accessible, dramatically expanding the geographic and demographic pool of potential participants. Learn more about this evolution in our article on Decentralized Clinical Trial Model.
The Future is Now: Leveraging Technology and AI in Recruitment
Clinical trial patient recruitment is undergoing a technological revolution. We are moving from manual chart reviews and broad-based advertising to data-driven strategies that use automation, real-world data (RWD), Electronic Health Records (EHRs), and predictive analytics to identify the right patients with remarkable precision.
Instead of casting a wide net, intelligent algorithms can pinpoint who might benefit from a study, making recruitment smarter, more effective, and more equitable. Technology and AI streamline every step, from identification and pre-screening to engagement and retention, dramatically reducing manual effort and accelerating timelines. For a comprehensive look at how these advances are reshaping our field, dive into our exploration of Current Systems and Technology in Clinical Trials.
The Role of Technology in Modern Clinical Trial Patient Recruitment
Technology is now the foundation of effective clinical trial patient recruitment. Patient matching algorithms can instantly analyze a patient’s entire medical profile against hundreds of complex trial criteria, identifying potential matches in seconds. These algorithms go beyond structured data fields (like diagnosis codes) by using Natural Language Processing (NLP) to scan unstructured text in clinician’s notes, pathology reports, and discharge summaries. This allows them to find nuanced criteria—such as disease progression or specific prior treatments—that would be impossible to find through manual review.
Digital prescreeners are another game-changer. These are intelligent, user-friendly online questionnaires that allow potential participants to assess their own eligibility at their convenience, 24/7. This self-service model respects the patient’s time, provides immediate feedback, and ensures that only the most promising candidates are forwarded to site staff, reducing screening failures and saving valuable resources.
Underpinning this innovation are secure data platforms that ethically integrate diverse data sources like EHRs, genomic databases, insurance claims, and even data from wearable devices. This creates richer, longitudinal patient profiles for more precise matching while upholding the highest standards of data privacy and governance. To see how these advances translate into practical improvements, explore our insights on Enhancing Clinical Trial Matching: Lifebit Patient Management.
How AI and Federated Learning Transform Data Access
Artificial Intelligence and Machine Learning are practical tools that spot complex patterns in massive datasets, uncovering potential trial participants that traditional methods would miss. However, a major challenge has been that valuable patient data is locked in separate, siloed systems across different hospitals and countries, unable to be pooled due to privacy regulations and data security concerns.
Federated data networks provide an elegant solution. Instead of moving sensitive patient data to a central location, the AI algorithms are sent to analyze the data where it resides. For example, a researcher could query three different hospital networks for patients with a specific EGFR mutation who have progressed on a certain therapy. The federated learning model is trained locally within each hospital’s secure firewall. The models learn from the data without any raw patient data ever leaving its secure environment. Only non-sensitive, aggregated insights or updated model parameters are shared back with the researcher.
This approach opens up access to previously unreachable real-world data, allowing us to generate real-time evidence on patient populations at a global scale. For clinical trial patient recruitment, this means we can identify eligible patients with unprecedented precision while ensuring data privacy and security. The implications are transformative: we can find rare disease patients across continents, build more robust and diverse cohorts, and identify underrepresented populations, all while rigorously protecting patient confidentiality. It’s about making trials more inclusive, efficient, and likely to succeed. To explore the full scope of how AI is revolutionizing clinical trials, check out our guide: AI for Clinical Trials.
Building Inclusive Trials: Strategies for Diversity and Special Populations
For too long, medical research has been dominated by narrow demographics, leaving entire communities underrepresented. This is not just an ethical issue; it’s a scientific one that impacts how well new treatments work in the real world. A drug tested primarily on one demographic may have different efficacy or side effect profiles in others.
Health equity is the foundation of meaningful medical progress. Recognizing this, regulatory bodies like the FDA now require sponsors to submit a Race and Ethnicity Diversity Plan early in clinical development. This is a formal demand for representative data.
The path forward requires a new approach to clinical trial patient recruitment. We must proactively build partnerships with diverse communities, working with patient advocacy groups, faith-based organizations, and local clinics to build trust and mutual respect. These collaborations help us understand and address the unique needs, cultural values, and logistical barriers of different communities. For a comprehensive look at how to break down these barriers, explore our guide on Breaking Barriers: Increasing Diversity in Clinical Trials.
Tailoring Recruitment for Rare Diseases and Niche Groups
Recruiting for rare diseases, where patients are few and geographically scattered, requires a creative, hyper-targeted approach. Patient advocacy groups are essential partners here. They are not just recruitment channels; they are the heart of their communities, providing support, education, and a powerful collective voice. Partnering with them early in the trial design process can ensure the protocol is patient-friendly and aligned with the community’s needs.
Genetic screening and decentralized trial elements open up new possibilities. Proactive, large-scale genomic screening programs can identify individuals with rare conditions before they are even symptomatic. Meanwhile, decentralized approaches eliminate the immense travel burdens that often make participation impossible. A patient in a rural area can participate in a trial run from a major research center thousands of miles away, thanks to telehealth, remote monitoring, and local lab partnerships. Key strategies include leveraging patient registries, using targeted social media where these communities gather, and adopting decentralized components to reduce logistical and financial burdens.
Measuring and Improving Diversity in Enrollment
Achieving diversity requires concrete goals, real-time tracking, and a willingness to adapt. Setting diversity goals at the outset of a trial, as now mandated by the FDA, is the first step. These goals should be based on the epidemiology of the disease, ensuring the trial population mirrors the real-world patient population.
Monitoring enrollment metrics in real time is crucial. This means tracking not just total numbers, but the demographic breakdown of screened, enrolled, and retained participants. If a trial’s demographics are not meeting targets, the recruitment strategy must be adjusted immediately. This could involve reallocating resources to sites in more diverse areas or launching new, targeted outreach campaigns.
Creating culturally competent materials and having bilingual staff goes beyond simple translation. It means using imagery, language, and examples that resonate with the target community. It means understanding cultural attitudes toward medicine and research. Most importantly, building trust with underrepresented communities is the critical element. This is earned through consistent, genuine engagement that extends beyond a single trial. It involves compensating participants fairly for their time and travel, providing clear communication about trial results (regardless of the outcome), and demonstrating a long-term commitment to the community’s health. Continuous optimization is key—treating diversity as an ongoing process of learning and adapting based on real feedback from communities.
Conclusion
Clinical trial patient recruitment is not just a procedural step; it is the foundation of medical research. Without enough participants, promising treatments remain stuck in the lab.
A patient-centric approach is the key to success. This means listening to patient concerns, understanding their motivations, and building trust through clear communication.
Technology and AI are critical enablers in this shift. Patient matching algorithms and digital prescreeners are making recruitment more precise and efficient. The most important development is the use of federated learning networks, which can securely analyze siloed patient data without moving it, opening up vast datasets while upholding the highest privacy standards.
Furthermore, diversity is non-negotiable. Trials must reflect the real-world populations they aim to serve to ensure treatments are effective for everyone. This is a matter of both scientific integrity and ethical responsibility.
At Lifebit, our federated platform is built to tackle these challenges. It enables secure, data-driven recruitment and analysis across global datasets, helping researchers connect with diverse patient populations while maintaining strict privacy. By leveraging rich, multi-omic data through our federated AI platform, we accelerate inclusive research that benefits everyone.
The future of recruitment is data-driven, patient-centered, and inclusive. With the right technology and approach, we can overcome the barriers that have long slowed medical progress.
Ready to see how our platform can transform your research efforts? Explore our federated data platform and find what’s possible when cutting-edge technology meets compassionate patient care.

