Your Comprehensive Guide to Rare Disease Registries and Beyond
The Scale and Significance of Rare Disease Registries
A rare diseases registry is a database that collects standardized information about individuals with the same rare condition to advance research and improve patient outcomes. These registries are critical bridges, turning individual experiences into powerful data that can lead to new treatments.
Key Facts About Rare Diseases:
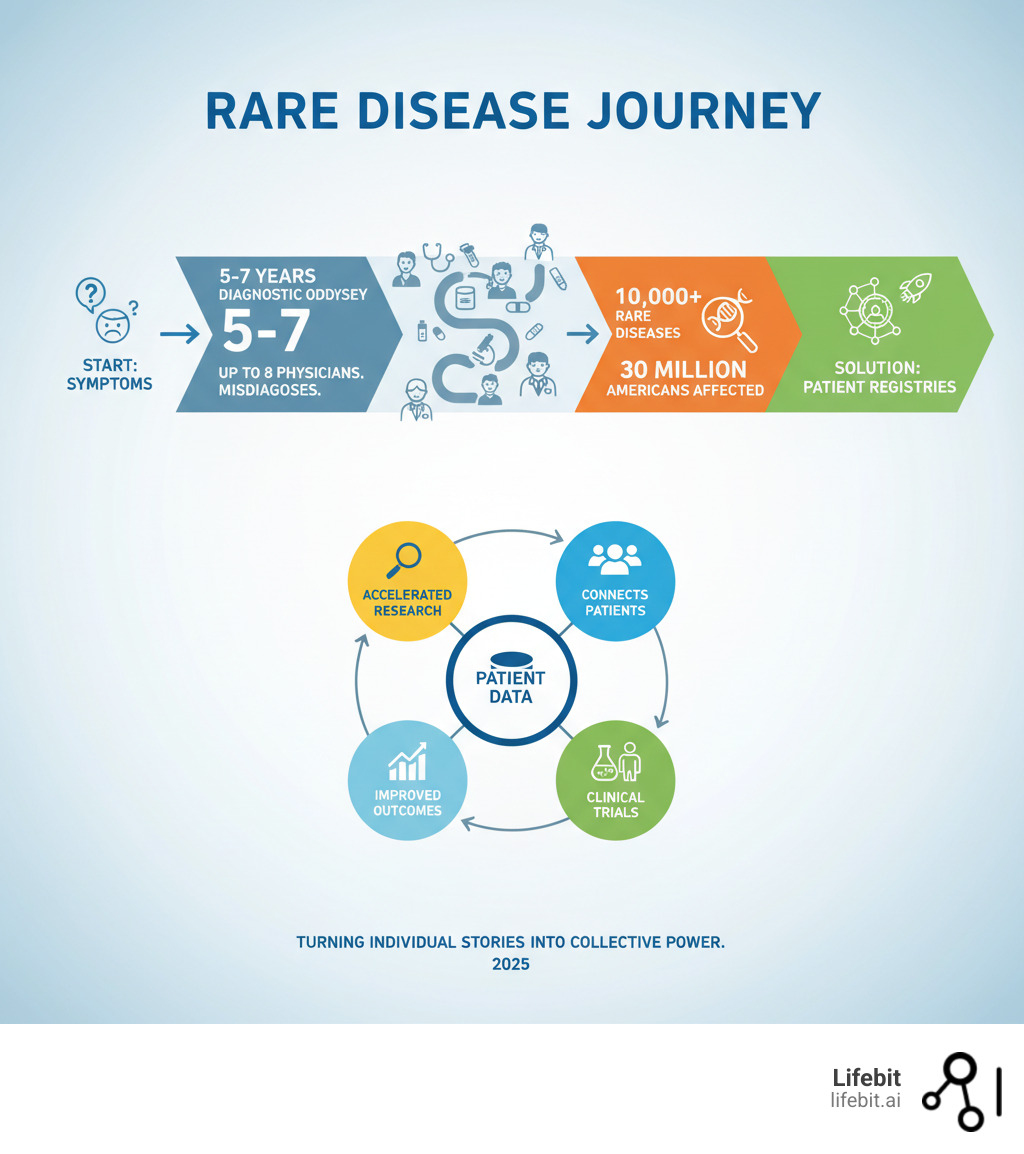
- 10,000+ known rare diseases exist worldwide
- 30 million Americans are affected
- 5-7 years average diagnostic odyssey
- 80% of rare diseases have a genetic component
- 200,000 people or fewer defines a “rare” disease in the US
When diseases are rare, finding enough patients for research is a major challenge. The more participants in a study, the more powerful the results. This is where rare diseases registry programs become game-changers, connecting patients with researchers and helping identify clinical trial participants. They provide the natural history data needed to understand how diseases progress, reshaping rare disease research.
As the CEO and Co-founder of Lifebit, I’ve spent over 15 years in computational biology and genomics, building platforms for secure analysis of sensitive biomedical data, including rare diseases registry information. Through federated data analysis, we help researchers open up insights from registry data without compromising patient privacy or moving data from its secure environment.
What Are Rare Disease Registries and Why Are They Crucial?
A rare diseases registry is an organized collection of information about people with the same uncommon medical condition. With fewer than 200,000 people affected by each condition in the US, traditional research struggles to gather enough data. Registries solve this by uniting scattered information from patients globally, creating a powerful resource for scientific progress. As we’ve explored in our article on Four Benefits of Patient Registries for Rare Diseases, these systems are indispensable for advancing research and enabling clinical trials.
To learn more about their foundational role, see our guide on What Are Patient Registries? Why Are They Important?.
How Registries Empower Patients and Caregivers
For families navigating a rare disease, a rare diseases registry offers connection, knowledge, and purpose, transforming an isolating experience into a communal effort. The psychological burden of a rare diagnosis is immense, often characterized by feelings of being alone. Registries break down these walls by creating a virtual community of individuals with shared lived experiences, fostering support networks and reducing feelings of isolation. Participants also gain access to curated, expert-vetted information about their condition, cutting through the noise of online misinformation. This is often facilitated through hubs like the NORD Rare Disease Database. Furthermore, by contributing data, patients and caregivers become active partners in the scientific process. They are no longer passive subjects but essential contributors whose data shapes the direction of research. As the Congenital Muscle Disease International Registry (CMDIR) notes, “Your voice and the data that you contribute can help identify and answer the questions that matter most.” This direct involvement ensures that research priorities—such as focusing on quality-of-life improvements versus purely clinical metrics—reflect the actual needs and values of the patient community, empowering them to influence the future of their own care.

How Registries Accelerate Research and Treatment
For researchers and drug developers, a rare diseases registry is the foundational tool that makes progress possible. It provides the critical mass of longitudinal, standardized data needed to overcome the inherent challenges of studying small, geographically dispersed populations.
Enabling Natural History Studies: A natural history study is a comprehensive, long-term observation of a cohort of patients to understand how a disease unfolds over time without a therapeutic intervention. For rare diseases, this is often the first and most critical step in research. Registries are the primary vehicle for these studies, collecting data on symptom onset, progression rates, and key milestones. This information is vital for regulatory bodies like the FDA and EMA, as it provides the baseline against which the effectiveness of a new drug can be measured. Without a clear understanding of a disease’s natural course, it’s impossible to prove a therapy is changing its trajectory.
Solving Clinical Trial Recruitment: One of the biggest barriers to developing new treatments for rare diseases is finding enough eligible patients to participate in a clinical trial. A disease might affect only a few thousand people worldwide, making traditional recruitment methods ineffective. Registries solve this by creating a centralized, pre-consented pool of potential participants. Researchers can query the registry to identify individuals who meet specific inclusion/exclusion criteria, dramatically accelerating the recruitment timeline from years to months. This is a core function of platforms like the RDCRN Contact Registry and CoRDS (Coordination of Rare Diseases at Sanford).
Establishing Meaningful Clinical Endpoints: To get a drug approved, researchers must demonstrate its effect on a specific, measurable outcome, known as a clinical endpoint. In well-understood diseases, these are established (e.g., blood pressure reduction). In rare diseases, appropriate endpoints are often unknown. Does the disease affect mobility, breathing, cognitive function, or a specific biomarker? Registry data, which captures a wide range of symptoms and patient-reported outcomes over time, is essential for identifying which metrics are most meaningful to patients and most responsive to change. This allows for the design of more efficient and relevant clinical trials.
Facilitating Genotype-Phenotype Correlations: With the integration of advanced genomic data, registries become even more powerful. By linking a patient’s genetic information (genotype) with their clinical symptoms and disease progression (phenotype), researchers can uncover crucial insights. They can identify how different genetic mutations lead to varying disease severities or subtypes, which can help in stratifying patients for trials and developing targeted therapies. This is a key area of focus in modern registry design, as highlighted in numerous studies on the utility of genome sequencing in rare disease cohorts.
For more on structured health data collection, explore our article on Disease Registers.
Types of Registries and Their Specific Goals
Not all registries are created equal; they are purpose-built to address different needs within the rare disease ecosystem. Understanding their specific goals is key to appreciating their collective impact.
Contact Registries: These are the simplest form of registry, acting primarily as a matchmaking service. Their goal is to collect basic contact and diagnostic information from patients who are willing to be contacted about future research opportunities. They are invaluable for researchers looking to quickly gauge interest or recruit for a wide range of studies, from simple surveys to complex clinical trials. The RDCRN Contact Registry is a prime example, serving as a central point of contact for a network of rare disease researchers.
Natural History Study Registries: These are far more comprehensive and are the backbone of rare disease research. Their purpose is to track the progression of a disease over an extended period. They collect detailed, longitudinal data, including medical history, genetic test results, symptoms, treatments, quality-of-life measures, and patient-reported outcomes. This rich dataset allows researchers to understand disease variability, identify prognostic biomarkers, and establish the clinical endpoints needed for trial design. NORD’s IAMRARE Program specializes in helping patient groups build these robust natural history registries.
Post-Market/Safety Registries: The work doesn’t stop once a treatment is approved. These registries are designed to monitor the long-term safety and real-world effectiveness of a new drug or medical device after it has entered the market. They collect data on side effects, adverse events, and long-term patient outcomes. This information is crucial for regulatory agencies (like the FDA and EMA), pharmaceutical companies, and healthcare providers to ensure the treatment’s benefits continue to outweigh its risks in a broader, more diverse patient population over many years.
Clinical Trial Registries: While the other registries support research broadly, these are built to support a specific clinical trial. They are designed to collect the precise data points required by the study’s protocol. Data collection is highly structured and often more rigorous, with defined visit schedules and specific assessments. While their focus is narrow, they are essential for executing a trial efficiently and collecting the high-quality evidence needed for regulatory submission. Often, data from a clinical trial registry may later be integrated into a broader natural history registry to enrich the long-term understanding of the disease.
Building and Managing a High-Quality Rare Diseases Registry
Creating a rare diseases registry connects isolated patients with researchers, turning scattered stories into a powerful resource that can change lives. Our guide, High-Quality Patient Registries: Establishment, details this transformative journey. A successful registry is built on good data practices, ethical methods, and a commitment to the community it serves.
Key Steps in Setting Up a Rare Diseases Registry
Building a rare diseases registry is a multi-faceted, strategic endeavor that moves from a foundational idea to a sustainable, long-term scientific resource. Each step requires careful planning and collaboration among diverse stakeholders.
1. Define Purpose and Scope: This is the most critical first step. Before any data is collected, the registry’s leaders must clearly articulate its primary goals. Is the main objective to conduct a natural history study to support drug development? Is it to connect patients with clinical trials? Or is it to monitor the safety of a new therapy? The purpose dictates the scope: what data to collect, from whom, and for how long. This phase involves extensive consultation with stakeholders—patients, caregivers, clinicians, researchers, and potential industry partners—to ensure the registry will meet their collective needs. Resources like the Guidance from the RaDaR program provide a structured framework for asking these foundational questions.
2. Secure Funding and Establish Governance: Registries are not one-time projects; they require sustained funding for development, maintenance, data curation, and participant engagement. Funding models vary widely. They can be secured through competitive government grants (e.g., from the NIH or European Commission), grassroots fundraising by patient advocacy groups, or direct partnerships with pharmaceutical companies interested in the data for their drug development programs. A hybrid model is often most sustainable. Alongside funding, establishing a clear governance structure—including a steering committee with patient representation—is essential for making transparent decisions about data access and research priorities.
3. Design the Registry and Data Collection Instruments: This is where the purpose is translated into practice. The team must decide exactly what information to collect using Case Report Forms (CRFs) and patient-reported outcome (PRO) questionnaires. The principle of ‘collecting the minimum data for the maximum impact’ is key to avoid overburdening participants. The design must consider longitudinal data collection (tracking changes over time) versus cross-sectional (a single snapshot). Using standardized Common Data Elements (CDEs) from the outset is crucial for future data sharing and interoperability.
4. Choose a Technology Platform: The registry’s software is its backbone. The decision often comes down to building a custom platform versus using an existing, configurable one. Modern platforms are typically cloud-based for scalability and accessibility, secure to protect sensitive data, and extremely user-friendly for patients who may be entering data from home on a mobile device. Key features include robust security, role-based access controls, data validation checks, and interoperability APIs. Platforms like NORD’s IAMRARE offer a turnkey solution for patient groups without deep technical expertise.
5. Obtain Ethical and Regulatory Approval: A registry is a form of human subjects research and therefore requires oversight from an Institutional Review Board (IRB) or an independent ethics committee. This is a non-negotiable step to protect participant rights and welfare. The IRB reviews the study protocol, data security measures, and, most importantly, the informed consent process. The consent form must clearly explain in plain language the registry’s purpose, what data will be collected, how it will be used and protected, the risks and benefits of participation, and the participant’s right to withdraw at any time.
6. Recruit and Engage Participants: With the framework in place, the focus shifts to building the community. Recruitment strategies must be multi-pronged, often leveraging the trusted voice of patient advocacy groups through their websites, social media channels, and newsletters. Collaboration with key clinical centers and specialists who see these patients is also vital. Recruitment is not a one-time event; ongoing engagement through regular updates, newsletters, and sharing of research findings is critical to retain participants and ensure high-quality, long-term data collection.
Data Quality, Standardization, and Privacy
For a rare diseases registry, the value of the data is directly proportional to its quality, consistency, and the trust participants place in its security. These three pillars—quality, standardization, and privacy—are intertwined and demand rigorous, ongoing attention.
Ensuring High Data Quality: The adage ‘garbage in, garbage out’ is especially true for registries. Major challenges include incomplete medical records, variability in how different clinicians describe symptoms, and recall bias in patient-reported data. To combat this, high-quality registries implement multiple layers of quality control. This includes built-in validation checks in the data entry forms (e.g., flagging impossible values), standardized questionnaires to reduce ambiguity, and automated error detection scripts. Many registries also employ professional data curators who review submitted data, follow up on missing or inconsistent entries, and may perform source data verification by comparing registry entries to actual medical records.
The Critical Role of Data Standardization and Interoperability: A single registry, no matter how well-designed, is often not large enough to provide definitive answers for an ultra-rare disease. The true power comes from the ability to combine or compare data across different registries. This is only possible through standardization. The use of Common Data Elements (CDEs)—standardized questions and definitions for collecting data, often developed by bodies like the NIH—is fundamental. Furthermore, mapping data to established biomedical ontologies is crucial. Ontologies are formal classification systems that provide a common language for describing concepts. Key examples include the Human Phenotype Ontology (HPO) for describing clinical abnormalities, Orphanet and MONDO for rare disease names and classifications, and SNOMED CT for clinical terms. Adhering to these standards makes the data FAIR—Findable, Accessible, Interoperable, and Reusable—which is the global benchmark for scientific data management.
Upholding Privacy, Ethics, and Trust: Trust is the currency of any patient registry. Participants are sharing their most sensitive health information and must be confident it will be protected. This is enforced through a combination of legal, ethical, and technical safeguards. Legally, registries in the US must comply with the Health Insurance Portability and Accountability Act (HIPAA), while those involving European citizens must adhere to the General Data Protection Regulation (GDPR), which has even stricter rules on data control and consent. Ethically, the informed consent process is paramount. Many registries now use a tiered consent model, allowing participants to choose precisely how their data can be used (e.g., only for academic research, or also for commercial research). Technically, data is de-identified by removing all 18 personal identifiers specified by HIPAA before it is shared with researchers. This creates a ‘safe harbor’ dataset that protects patient identity. Advanced platforms like Lifebit’s federated system take this a step further, enabling analysis of registry data without it ever leaving its protected environment, offering the highest possible standard of privacy while maximizing collaborative potential.
The Ecosystem: Key Organizations and Patient Power
A successful rare diseases registry requires a village—a vibrant ecosystem of patients, caregivers, clinicians, researchers, and industry partners. This collaborative approach, emphasized by the RaDaR program, combines the lived experiences of patients with the scientific expertise of researchers and the resources of industry partners to achieve what no single group could alone.
The Role of Patient Advocacy Groups
Patient advocacy groups are often the driving force behind rare diseases registry programs. These passionate organizations, founded by families and individuals, are instrumental in creating and funding registries, mobilizing communities, and building support networks. They ensure the patient voice remains central to research, championing patient-reported outcomes and aligning research priorities with what truly matters to those living with a condition. Groups like the BOS Foundation, Cystinosis Research Foundation, GNB1 Advocacy, and National Ataxia Foundation have transformed the landscape for their communities.
How Organizations like NORD, NCATS, and RDCRN Provide Support
While patient groups provide passion, national organizations offer the infrastructure and expertise to build world-class registries.
- NORD’s IAMRARE® Program: This platform allows patient groups to launch high-quality, customized registries without deep technical knowledge. It provides a cloud-based, mobile-friendly system designed to collect the natural history data crucial for treatment development. More about the IAMRARE® Program.
- NCATS’ RaDaR Program: RaDaR offers comprehensive guidance and resources for building registries, providing step-by-step instructions on legal, ethical, and standardized methods for data collection and sharing.
- The Rare Diseases Clinical Research Network (RDCRN): This NIH-funded network of 20 consortia facilitates large-scale collaborative research. Its Contact Registry connects patients to clinical trials and promotes information sharing across the rare disease community.
Together, these organizations provide the framework and collaborative spirit that allow rare diseases registry initiatives to flourish, accelerating the path to life-changing treatments.
Technology and the Future of Rare Disease Research
The world of rare disease research is undergoing a profound transformation, with technology as the primary catalyst. Rare diseases registry programs are evolving from static databases into dynamic, intelligent ecosystems that can integrate diverse data types and extract insights at an unprecedented scale. This technological leap is not just making research faster; it’s making it smarter and more patient-centric.
Mobile-friendly platforms and wearable technology are democratizing data contribution, allowing patients to share information continuously from their own homes. This shift from episodic, in-clinic data collection to real-time, longitudinal monitoring provides a much richer picture of a disease’s impact on daily life. The integration of genomic data remains a cornerstone of this evolution. As our exploration of Rare Disease Diagnosis & Genomics shows, linking whole-exome or whole-genome sequences with detailed clinical data within a registry is the key to unlocking genotype-phenotype correlations and identifying novel therapeutic targets.
Perhaps the most exciting frontier is the application of AI and machine learning to registry data. These advanced algorithms can detect subtle patterns in vast, complex datasets that are impossible for humans to discern. They can be used to stratify patients into distinct subgroups based on disease progression, predict future clinical outcomes, and even identify previously undiagnosed individuals by scanning electronic health records for tell-tale symptom clusters. At Lifebit, our federated AI platform is specifically designed for this type of advanced analysis, allowing researchers to deploy sophisticated machine learning models on sensitive rare diseases registry data while ensuring patient information remains completely secure within its original, protected environment.
Enhancing Registries with Modern Software
Modern rare diseases registry software is no longer a simple data repository; it’s a comprehensive research management suite. Cloud-based platforms, such as the one offered by NORD’s IAMRARE Program, provide the essential foundation of scalability, global accessibility, and robust security. But the real power lies in a suite of intelligent features designed to maximize data quality and research utility. For example, automated reminders and patient-friendly dashboards significantly improve participant retention and the completeness of longitudinal data. Integrated data visualization tools empower researchers and even patient groups to explore trends and generate hypotheses directly within the platform, turning raw data into actionable insights without needing a separate team of analysts. Crucially, sophisticated role-based permissioning and audit trails ensure that different users (patients, coordinators, researchers) can only access the specific data they are authorized to see, enabling secure collaboration across institutions. These features, which we detail in Patient Registry Software and Four Key Patient Registry Software Requirements, are what transform a registry from a passive database into a powerful engine for discovery.
The Role of a Rare Diseases Registry in a Connected Health Data Ecosystem
The future of medical research lies not in isolated datasets but in a connected global health data ecosystem. A rare diseases registry is a critical node in this network, breaking down the silos that have historically separated different types of health information. The true breakthrough comes from linking high-quality, patient-provided registry data with other sources, such as electronic health records (EHRs), genomic and multi-omic data, and even insurance claims data. This linkage creates a holistic, 360-degree view of the patient journey.
This integrated dataset forms the basis of Real-World Evidence (RWE)—evidence regarding the usage and potential benefits or risks of a medical product derived from the analysis of Real-World Data (RWD). Regulatory agencies like the FDA and EMA are increasingly accepting RWE to support drug approvals, especially for rare diseases where traditional, large-scale randomized controlled trials are not feasible. A registry provides the longitudinal clinical context that makes other data sources, like a one-time genomic test or fragmented EHR entries, scientifically meaningful.
This connected approach is essential for enabling global collaboration, which is non-negotiable for diseases so rare that a sufficient number of patients can only be found by pooling data across continents. However, this creates immense challenges around data privacy and sovereignty, as regulations like GDPR often prohibit sensitive patient data from leaving its country of origin. This is where federated technology provides a solution. Lifebit’s federated AI platform is built for this connected future. Instead of moving data to a central location for analysis, our platform sends the analytical tools and machine learning models to the data, wherever it resides. The analysis is performed locally, behind the institution’s firewall, and only the aggregated, non-identifiable results are returned. This ensures that sensitive rare diseases registry information never leaves its secure environment. Researchers can therefore securely analyze registry data in combination with other global health datasets, unlocking insights faster and more safely than ever before with tools like our Trusted Research Environment and Real-time Evidence & Analytics Layer.
Frequently Asked Questions about Rare Disease Registries
Sharing medical information is a big decision. Here are answers to common questions about rare disease registries.
What is the main purpose of a rare disease registry?
The main purpose of a rare diseases registry is to collect standardized information from people with the same rare condition. This data helps researchers understand the disease’s natural history (how it progresses), connects patients with clinical trials, and ultimately accelerates the development of new treatments by providing a comprehensive picture of the disease.
How can I find and join a registry for my condition?
Finding the right registry is easier than you might think.
- Start with patient advocacy groups for your specific condition, as they often run or partner with registries.
- Explore resources from major organizations like NORD (National Organization for Rare Disorders) and its IAMRARE® Program.
- Check clinical trial databases like ClinicalTrials.gov or the RDCRN Contact Registry.
- Talk to your healthcare team, including specialists and genetic counselors, who may know of relevant research opportunities.
Is my personal information safe in a registry?
Yes, reputable registries take privacy very seriously. Your information is protected in several ways:
- Legal Protections: Registries must comply with strict privacy laws like HIPAA in the United States.
- Informed Consent: You must be given clear information on how your data will be used and protected before you agree to participate. You can withdraw at any time.
- Data De-identification: Before data is shared with researchers, all personal identifiers (name, address, etc.) are removed to protect your identity.
- Secure Storage: Information is stored using industry-standard security measures like encryption and access controls.
At Lifebit, our federated platform adds another layer of security, allowing researchers to analyze sensitive rare diseases registry data without it ever leaving its secure environment.
Conclusion
Rare diseases registry programs represent a powerful shift in medicine, turning the isolated experiences of millions into a connected movement that accelerates scientific findy. Every patient who contributes their data adds a crucial piece to the puzzle, helping researchers solve the challenges of rare disease.
For patients, registries offer community, access to clinical trials, and the power to shorten the long diagnostic odyssey. For researchers, they are goldmines of insight that make natural history studies and clinical trial recruitment more efficient. This progress is driven by a collaborative spirit, uniting patient advocacy groups with major organizations like NORD, NCATS, and the RDCRN.
Technology is making the future even brighter. Modern registry platforms, integrated with genomic data and powered by AI, are uncovering insights faster than ever.
At Lifebit, we are proud to support this movement. Our federated AI platform enables researchers to analyze sensitive rare diseases registry data without compromising patient privacy, ensuring insights can travel the world even when the data cannot. The future for rare disease patients is hopeful, as the collective power of these initiatives proves that no challenge is too rare to overcome.
Explore Lifebit’s federated platform for secure research to see how we’re helping connect and analyze vital health data for rare disease research and beyond.

