Patient Recruitment in Clinical Trials: Your Essential Handbook

Why Patient Recruitment in Clinical Trials Makes or Breaks Drug Development
Patient recruitment in clinical trials is where most trials fail. Research shows 76% of randomized clinical trials stop early due to poor recruitment, and only 55% hit their targets. In the U.S., over 80% of trials miss enrollment deadlines, causing delays that cost companies $1.2 billion USD per trial and consume up to 30% of development timelines.
What you need to know about patient recruitment in clinical trials:
- Recruitment = identifying and sourcing patients up to informed consent signing
- Enrollment = the full funnel from pre-screening through randomization
- Key challenges = strict eligibility criteria, lack of awareness, patient burden, geographic barriers
- Proven strategies = multi-channel outreach, site-specific planning, patient-centric design, technology integration
- Critical for success = clear metrics tracking, retention focus, diversity planning, ethical compliance
This guide covers every stage of patient recruitment. You’ll learn the difference between recruitment and enrollment, how to use AI and Real-World Data for faster patient identification, and how to build retention strategies that work. Find why old methods fail and how to design trials that patients want to join.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. We build federated data platforms that help pharma and public health institutions analyze diverse health data (EHR, genomics, claims) without moving it. This directly solves the data access and patient identification challenges that slow patient recruitment in clinical trials. My work in computational biology has shown me how data-driven approaches can turn recruitment from a bottleneck into a competitive advantage.
Relevant articles related to patient recruitment in clinical trials:
- AI clinical trial recruitment
- clinical trial patient recruitment companies
- clinical trial patient support
The Foundation: Distinguishing Recruitment from Enrollment
Even experienced trial managers confuse patient recruitment and patient enrollment. The distinction isn’t just semantics—it’s the key to diagnosing why your trial is losing participants and where your budget is being wasted.
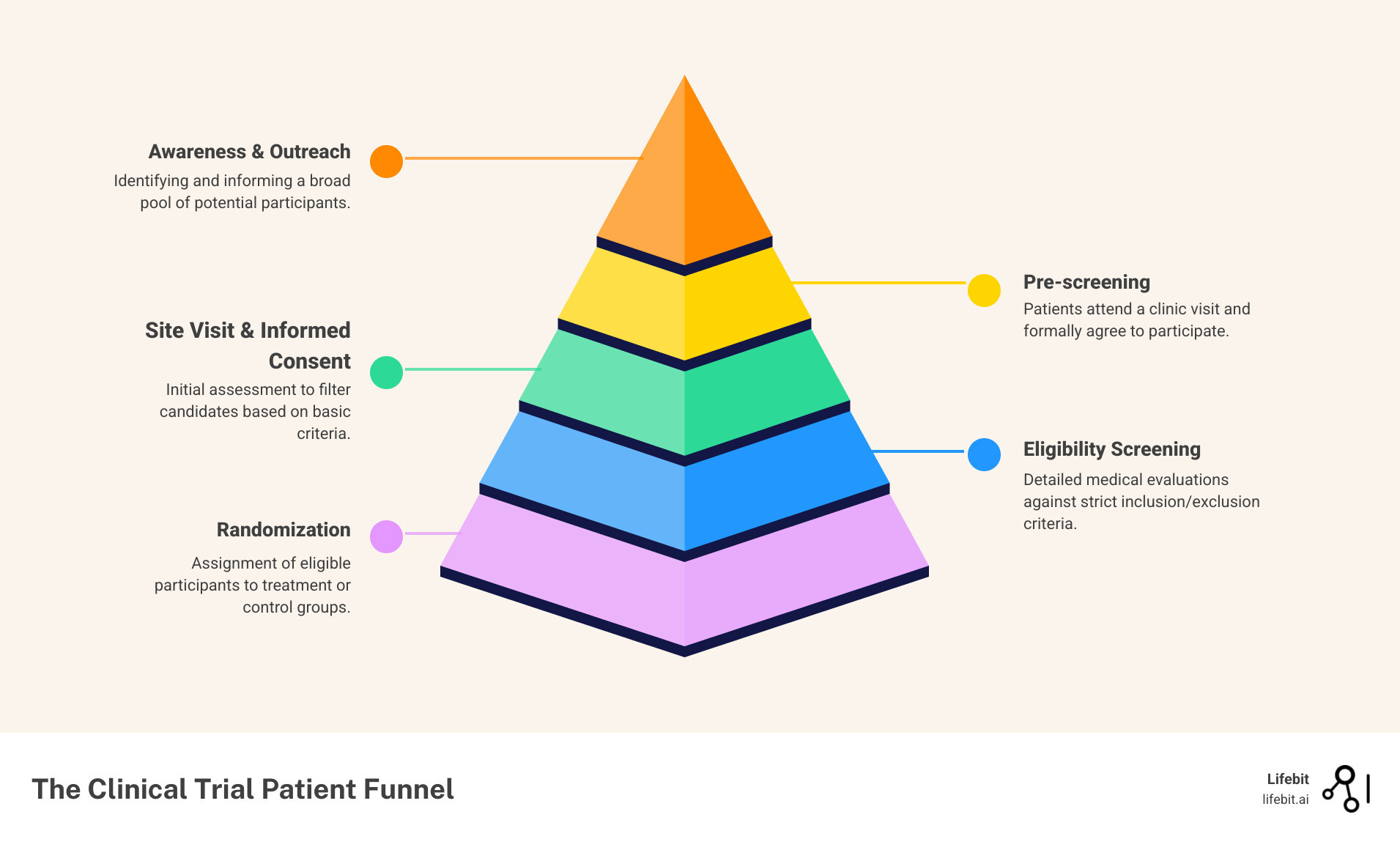
Patient recruitment covers everything up to the point a participant signs the informed consent form. It includes identifying potential candidates, conducting outreach, performing initial pre-screening to gauge interest and basic eligibility, and educating them about the trial. Think of it as the courtship phase: building awareness, generating interest, and helping someone decide if the trial is a good fit for them.
Patient enrollment is the entire funnel, from identification through randomization. After a patient is recruited and signs the consent form, they enter the screening phase. Here, they undergo formal assessments to confirm they meet all of the study’s detailed inclusion and exclusion criteria. Only after passing this rigorous screening are they officially assigned (randomized) to a treatment group. A patient is only considered fully enrolled once they are randomized into the study. Many who are recruited never get enrolled, which is why this distinction is critical.
Why This Distinction Is Critical for Trial Success
Understanding this difference gives you X-ray vision into your patient funnel, allowing for precise, data-driven interventions.
- Surgical Bottleneck Identification: If you have high traffic to your trial landing page but few people complete the pre-screener, it’s a recruitment messaging problem. If many people pass the pre-screener but never sign consent, it could be a site follow-up issue. If they sign consent but a high percentage fail formal screening, it’s an enrollment problem, likely pointing to overly strict eligibility criteria or a mismatch between your recruitment targeting and the protocol requirements.
- Granular Tracking and KPIs: Instead of one vague “enrollment rate,” you can track a cascade of metrics: recruitment velocity (leads per week), consent rates (consented/pre-screened), and screening pass rates (randomized/consented). This helps you spot trends early, like a specific site that’s great at recruiting but poor at screening, and intervene before timelines are compromised.
- Data-Driven Resource Allocation: High pre-screening failure rates suggest your advertising or outreach channels are targeting the wrong audience. Low screening success rates mean you should revisit the protocol’s eligibility criteria with the sponsor. This stops you from wasting budget on generic “do more” strategies and focuses resources where they will have the most impact.
- Improved Financial Forecasting: Confusing recruitment with enrollment leads to flawed budget projections. The cost per recruited patient is vastly different from the cost per enrolled (randomized) patient. By tracking these separately, you can accurately model the true cost of your trial and avoid unexpected financial shortfalls.
- Actionable Site Performance Analysis: When sponsors and sites use the same definitions, conversations shift from blame (“Your site isn’t enrolling!”) to collaborative problem-solving based on clear data (“Your site has a great consent rate, but we’re seeing a 70% screen-fail rate on criterion X. Let’s discuss why.”).
- Improved Sponsor-Site Communication: Clear definitions lead to clear expectations, better reporting, and faster solutions. No more confusion over whether a site’s “enrolled” number means “signed consent” or “randomized.”
Actionable Metrics for Recruitment and Enrollment
Track different metrics at each stage to manage patient recruitment in clinical trials effectively.
For recruitment, focus on:
- Referral source effectiveness: Track cost-per-lead and cost-per-qualified-lead by channel (e.g., social media ads, HCP referrals, patient advocacy groups) to optimize budget allocation.
- Site follow-up speed: Measure the time from when a lead is sent to a site to the first contact. Delays of more than 24-48 hours are a primary cause of patient drop-off.
- Pre-screening failure rates: Analyze why patients are failing the initial screener. Are the questions unclear? Is your targeting off?
- Awareness reach and engagement: Monitor impressions, click-through rates, and engagement on digital campaigns to ensure your message is reaching a sufficiently large and relevant audience.
For enrollment, zero in on:
- Inclusion/exclusion (I/E) criteria failures: Track the specific I/E criteria that are disqualifying the most patients during formal screening. This data is crucial for protocol amendments.
- Reasons for consent withdrawal: If patients sign consent but then walk away before randomization, find out why. Was the screening process too burdensome? Did they have second thoughts?
- Screen-to-randomization ratio: This key performance indicator (KPI) reveals the efficiency of your screening process. A low ratio indicates a problem with your protocol design or recruitment targeting.
- Enrollment velocity: Measure the number of patients randomized per site per month. Compare this against the planned timeline to forecast potential delays.
| Feature | Patient Recruitment | Patient Enrollment |
|---|---|---|
| Definition | Identifying, sourcing, educating patients for pre-screening and initial discussions up to informed consent. | The entire process from initial identification through to randomization. |
| Key Stages | Awareness, Initial Contact, Pre-screening, Informed Consent Discussion, Consent Form Signing. | All recruitment stages, plus Formal Screening, Eligibility Confirmation, Randomization. |
| Primary Goal | Generate a pool of interested, potentially eligible candidates ready for screening. | Successfully integrate qualified participants into the study. |
| Actionable Metrics | Referral source effectiveness, Site follow-up speed, Pre-screening failure rates. | I/E criteria failure rates, Reasons for decline, Screen-to-randomization ratio, Enrollment velocity. |
| Focus | Outreach, education, initial qualification. | Rigorous qualification, retention through screening, study entry. |
| Outcome | Signed Informed Consent. | Randomized participant. |
The Crisis: Why 76% of Trials Fail at Patient Recruitment
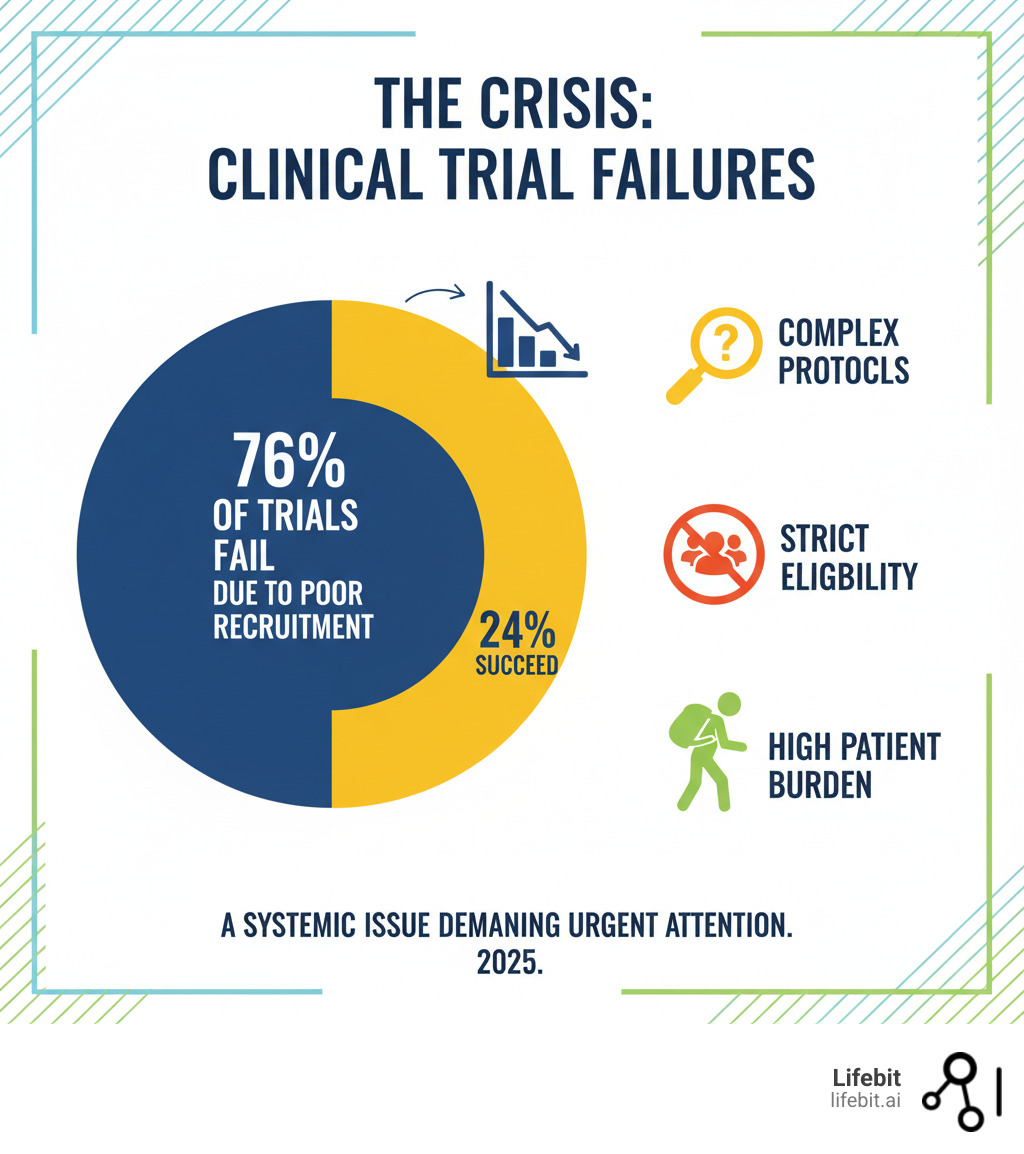
The hard truth is that 76% of randomized clinical trials are discontinued or stop early because they can’t find enough patients. This staggering statistic represents billions of wasted dollars, stalled scientific progress, and, most importantly, patients left waiting for potentially life-saving new treatments. In the U.S., over 80% of trials miss their enrollment targets, creating a systemic crisis that costs the industry an estimated $1.2 billion USD in lost revenue per trial and consumes up to 30% of drug development timelines.
So why is patient recruitment in clinical trials so profoundly difficult? The same core challenges appear again and again, creating a perfect storm of inefficiency.
- Protocol complexity: Modern trial protocols have become increasingly intricate. A typical protocol might require 15 site visits over six months, multiple invasive biopsies, frequent blood draws, and daily electronic diary entries. For site staff, this translates to a heavy administrative burden—coordinating appointments, managing complex data entry, and ensuring adherence to hundreds of procedural steps. For patients, it means significant disruption to their work, family, and personal lives, which can be a powerful disincentive to participation.
- Overly strict eligibility criteria: While designed to ensure scientific rigor and patient safety, inclusion/exclusion (I/E) criteria often become a primary, self-inflicted bottleneck. It’s not uncommon for criteria to eliminate over 95% of the real-world patient population. Common examples include narrow age ranges (e.g., 18-55) for diseases that also affect older populations, unrealistic BMI requirements, or the exclusion of patients with common comorbidities like controlled hypertension or type 2 diabetes—conditions often prevalent in the target disease population. Another major hurdle is the prohibition of concomitant medications; a patient who is otherwise a perfect fit might be excluded for taking a common antidepressant or a statin. This not only makes recruitment nearly impossible but also limits the generalizability of the trial’s findings to the diverse patients who will ultimately use the drug.
- Patient burden: Participation is a significant commitment. It means time off work for appointments, travel costs, and the physical and emotional toll of procedures. The financial strain is not trivial; a study by the Tufts Center for the Study of Drug Development found that out-of-pocket costs for trial participants can average thousands of dollars for travel, accommodation, and lost wages. With over two-thirds of Americans living more than two hours from the nearest major academic research center, the logistical and financial burdens are often insurmountable.
- Lack of awareness and education: The problem is deeper than disinterest; it’s a fundamental knowledge gap. A survey by the Center for Information and Study on Clinical Research Participation (CISCRP) found that 81% of the U.S. population had never heard of basic research protections like the Declaration of Helsinki or Institutional Review Boards (IRBs). The same study found that after being educated on these safeguards, nearly 40% of respondents became more willing to participate, highlighting the power of education in building trust.
- Geographic barriers: The traditional site-based trial model inherently excludes a massive portion of the population. For those in rural areas or with mobility issues, the nearest research center can be a day’s journey away, making consistent participation impossible.
- Mistrust: Historical abuses in medical research, such as the Tuskegee Syphilis Study, have created deep-seated and justified wariness in many communities, particularly among racial and ethnic minorities. Patients often worry about being treated as “guinea pigs” or being placed in a placebo group without receiving any benefit. These are valid concerns that must be addressed with transparency, community engagement, and a demonstrable commitment to ethical conduct.
- Budgetary constraints: Despite being a critical determinant of success, recruitment is often underfunded. Trial sponsors may allocate insufficient funds for outreach, site support, and patient reimbursement, leaving even well-designed trials without the resources needed to execute an effective recruitment strategy.
These factors combine to make patient recruitment a major bottleneck in drug development. However, understanding these challenges in detail is the first step toward designing effective solutions.
Modern Strategies for Successful Patient Recruitment in Clinical Trials
The recruitment crisis is solvable, but it requires a new playbook. Relying on clinic flyers, a single recruitment channel, and a one-size-fits-all message no longer works. A successful modern approach is holistic, data-driven, and relentlessly patient-centric, integrating multiple strategies to create a robust recruitment engine.
- Multi-channel approach: Meet patients where they are. This means deploying a coordinated strategy that includes digital advertising on social media and search engines, collaborations with patient advocacy groups, referrals from healthcare providers, and engagement through online health communities.
- Site-specific strategies: A rural community hospital faces entirely different recruitment challenges than a major urban academic center. Successful recruitment requires tailoring plans to local demographics, healthcare infrastructure, and referral networks. This includes providing sites with customized outreach materials and support based on their unique patient population.
- Data-driven planning and execution: The process must start with evidence-based feasibility assessments to design realistic eligibility criteria and enrollment timelines. Once the trial is active, real-time monitoring of recruitment metrics is essential to identify bottlenecks and adapt your strategy quickly.
- Community engagement: Building trust is the foundation of successful recruitment, especially in underrepresented populations. While traditional methods like personal referrals from trusted physicians remain highly effective, digital methods are essential for expanding reach. The key is to integrate both, using technology to support and scale community-based efforts.
Using Technology and Data to Find the Right Patients
Technology is transforming recruitment from a manual, often inefficient process into a smarter, more precise, and faster operation.
AI-powered trial design and patient identification analyzes vast datasets (genomics, EHRs, claims) to model the impact of different protocol criteria, helping sponsors design more inclusive trials from the start. During recruitment, machine learning algorithms can scan millions of patient records in minutes to pinpoint eligible candidates with high accuracy, replacing months of laborious manual chart review. This technology can also predict recruitment challenges and personalize outreach, significantly boosting enrollment rates.
At Lifebit, our federated AI platform uses AI/ML to identify patients from diverse, distributed data sources without moving sensitive data. This overcomes the privacy and security barriers that have historically blocked access to the rich, multi-modal data needed to revolutionize recruitment.
Real-World Data (RWD) from sources like Electronic Health Records (EHRs), insurance claims, and disease registries provides invaluable intelligence for trial planning and execution. Analyzing RWD helps sponsors understand disease prevalence, patient demographics, and standards of care in different regions. This intelligence is critical for designing inclusive trials, selecting the right sites, and targeting recruitment efforts with precision. Our federated technology enables the secure, real-time analysis of this distributed data.
Electronic Health Records (EHRs) have become a primary recruitment tool for about 50% of investigators. EHR-based alerting systems can notify physicians in real-time when one of their patients may be eligible for a trial, turning the point of care into a powerful recruitment opportunity.
Natural Language Processing (NLP) is a game-changer because it unlocks the 80% of clinical data that is unstructured, such as physician’s notes, pathology reports, and discharge summaries. NLP algorithms can interpret this text to identify patients who meet complex criteria that structured data fields (like diagnosis codes) would miss.
Digital advertising on platforms like Google, Facebook, and specialized health communities allows for precise targeting of individuals who are actively seeking treatment options or information about their condition. With careful strategy, culturally competent messaging, and performance monitoring, these campaigns can reach large, diverse populations effectively and cost-efficiently.
The future is federated technology that enables secure analysis of distributed datasets. It respects patient privacy and data ownership while maximizing insight—a win-win that centralized data models, which require risky data transfers, cannot offer.
Building a Patient-Centric Ecosystem for Better Patient Recruitment in Clinical Trials
Finding patients is half the battle; creating an experience that makes them want to join and stay is the other.
- Patient advocacy groups: These organizations are invaluable partners. Involving them early in the design process helps create patient-friendly protocols. During recruitment, they can raise awareness through their trusted channels and provide ongoing support to participants, which significantly improves retention.
- Healthcare provider (HCP) referrals: Doctors remain the most trusted source of health information for patients. Engaging and educating HCPs about ongoing trials and making the referral process seamless (e.g., through EHR alerts or simple online portals) is one of the most effective ways to accelerate recruitment.
- Patient-centric trial design: Involve patients or patient advocates in the protocol design process. Their input on the feasibility of visit schedules, the burden of procedures, and the clarity of patient-facing materials is invaluable for reducing participant burden and boosting engagement.
- Minimizing patient burden: This is a practical necessity. Offer flexible scheduling, provide transportation assistance or reimbursement, and ensure participants are fairly compensated for their time. Exploring decentralized trial (DCT) models that use technology to reduce or eliminate the need for site visits is a powerful way to remove geographic barriers.
- Clear and compassionate communication: All patient-facing materials, from advertisements to informed consent forms, should be written in simple, jargon-free language (aiming for a 7th-8th grade reading level). Trust built through transparent, empathetic, and consistent communication is a cornerstone of both recruitment and retention.
How to Integrate Diversity and Inclusion into Your Recruitment Strategy
Therapies must be proven safe and effective for everyone, but clinical trials have historically and systematically underrepresented diverse populations. This is not just an ethical failure; it’s a scientific one. In 2021, racial and ethnic minorities made up 39% of the U.S. population but accounted for only 2-16% of participants in trials for new drugs. Since different genetic and social factors can cause groups to respond differently to treatments, testing on a narrow, homogenous demographic leads to incomplete science and can exacerbate health disparities.
Regulators are taking action. The FDA now requires Diversity Action Plans for most late-stage trials, mandating that sponsors outline specific goals and strategies for enrolling underrepresented groups. The goal isn’t just compliance; it’s that representative trials lead to better, more generalizable science.
Achieving health equity in clinical research requires a deliberate, multi-faceted approach:
- Targeted outreach and partnerships: Go beyond standard channels. Partner with trusted community organizations, faith-based groups, community health centers, barbershops, and local media outlets that serve minority communities. Co-develop culturally sensitive messaging that addresses specific concerns and highlights the value of participation for the community.
- Building trust through long-term engagement: Trust cannot be built overnight. It requires establishing a continuous, respectful presence in communities, not just showing up when you need participants. This can involve sponsoring local health fairs, providing health education, and creating Community Advisory Boards to ensure research is relevant and respectful to the community’s needs and values.
- Cultural competency and training: All study team members, from investigators to front-line coordinators, must receive training on cultural competency, implicit bias, and the historical context of mistrust in medical research. This ensures that interactions with all potential participants are respectful, empathetic, and effective.
- Strategic site selection and accessibility: Expand trial sites beyond major academic centers into community-based clinics and rural health centers that serve diverse populations. Reduce logistical barriers by providing transportation, childcare support, and flexible appointment times. Leveraging decentralized trial components can also dramatically improve access for underrepresented groups.
Beyond Recruitment: Mastering Patient Retention
A brilliant recruitment strategy is useless if participants drop out before the trial is complete. Retention—keeping participants engaged, compliant, and active from start to finish—is the other half of the battle. High dropout rates, known as attrition, compromise data integrity, threaten study validity, and can derail a research program. With the average U.S. trial attrition rate hovering around 30%, mastering retention is not optional; it’s essential.

The Financial and Scientific Cost of Attrition
Every dropout carries a significant cost. Financially, the entire investment in recruiting and screening that participant—which can range from $5,000 to over $20,000—is lost. Scientifically, the consequences are even more severe. High attrition can compromise the study’s statistical power, meaning the trial may no longer have enough data to reliably detect a treatment effect. This can render the results inconclusive, forcing sponsors to extend recruitment timelines and enroll additional patients, which further inflates costs and delays the delivery of new therapies to the public.
Top Strategies for Enhancing Patient Retention
Retention begins the moment a patient expresses interest and is built on a foundation of trust, communication, and respect. It’s about creating an experience that makes participants feel like valued partners in the research process.
- Personalized and Proactive Communication: Don’t let participants feel forgotten between visits. Use a multi-touch communication plan that includes automated appointment reminders, newsletters with general study milestones (without unblinding data), and a dedicated contact person for questions. This makes participants feel connected to the study’s purpose.
- Flexible & Remote Monitoring (Decentralized Trials): Reduce the burden of travel and time off work by bringing the trial to the patient. This is the core principle of Decentralized Clinical Trials (DCTs), which leverage technology like telemedicine for virtual visits, wearable sensors for continuous data collection, mobile apps for electronic Patient-Reported Outcomes (ePRO), and partnerships with local labs or home health nurses for sample collection. By dramatically reducing the need for on-site appointments, DCTs make continued participation far more feasible for a wider, more diverse patient population.
- Meaningful Support & Ethical Incentives: Address the practical barriers to participation. Provide ethically approved incentives and fair reimbursement for time and travel. Partnering with patient advocacy groups can provide an extra layer of emotional and logistical support. Where appropriate and allowed by the protocol, providing participants with access to their own health data collected during the trial can be a powerful engagement tool.
- Operational Excellence at the Site Level: A smooth operational process is critical. This starts with comprehensive training for site staff, focusing not just on the protocol but on soft skills like empathetic communication and cultural competency. Providing patients with clear visit guides and checklists reduces confusion. Most importantly, streamlining the reimbursement process is critical; using reloadable debit cards or automated payment systems ensures patients are not out-of-pocket for extended periods, a common source of frustration and a key driver of attrition.
- Cultivating a Positive Site Experience: The relationship between the participant and the site staff is one of the strongest predictors of retention. A welcoming, respectful environment where participants feel heard and valued is a powerful retention tool. Simple acts of appreciation and a strong rapport with the study coordinator can make all the difference.
Retention in patient recruitment in clinical trials isn’t an afterthought—it’s a continuous commitment to the patient experience that begins with the first interaction and extends beyond the final visit.
The Guardrails: Ethical and Regulatory Frameworks
Ethical and regulatory frameworks are the essential guardrails for patient recruitment in clinical trials. They are not bureaucratic hurdles but the very foundation of trustworthy research, designed to honor participant autonomy, protect their wellbeing, and maintain scientific integrity.
- The Informed Consent Process: This is the cornerstone of ethical research. It is an ongoing dialogue, not just a signature on a form. The process must ensure participants fully understand the study’s purpose, procedures, potential risks, possible benefits, available alternatives, and their unequivocal right to withdraw at any time without penalty. Information must be presented in simple, non-coercive language (e.g., 8th-grade reading level). The rise of digital technology has introduced electronic informed consent (eConsent), which uses interactive multimedia like videos and quizzes to improve comprehension. eConsent platforms also facilitate remote consenting for decentralized trials and provide a clear audit trail to document that the process was completed correctly.
- Institutional Review Board (IRB) / Ethics Committee (EC) Review: Before a single patient can be contacted, an independent IRB or EC must review and approve the entire study protocol and all recruitment materials. IRBs conduct different levels of review based on risk: studies with minimal risk may get an expedited review, while those with greater risk (e.g., testing a novel drug) require a full board review. Their oversight covers advertisements, websites, pre-screening questionnaires, and consent forms to ensure they are accurate, balanced, and not coercive.
- Advertising and Recruitment Material Regulations: Regulatory bodies like the FDA have strict rules for trial advertising. Materials cannot make explicit or implicit claims that the investigational drug is safe or effective. They must clearly state that the product is investigational and that the activity is research, not standard clinical treatment. Language that could be seen as guaranteeing a positive outcome or cure is strictly prohibited.
- Avoiding Therapeutic Misconception: This common issue arises when participants believe that the primary purpose of the trial is to provide them with personalized treatment, rather than to produce generalizable scientific knowledge. Clear, consistent, and empathetic communication about the research nature of the trial, the possibility of receiving a placebo, and the uncertainty of the outcomes is the best defense against this misconception.
- Data Privacy and Confidentiality: Global regulations set strict rules for handling personal health information. In the U.S., the Health Insurance Portability and Accountability Act (HIPAA) requires specific patient authorization to use their Protected Health Information (PHI) for research. In Europe, the General Data Protection Regulation (GDPR) imposes even stricter requirements, mandating a clear legal basis for data processing and granting individuals rights like the ‘right to be forgotten.’ Navigating these complex regulations is a major challenge, especially for global trials. This is where federated technology, like the platform offered by Lifebit, provides a critical solution. By allowing analysis to happen where the data resides, it enables the use of sensitive health data for recruitment without exposing or moving the raw data, thus inherently complying with the core principles of data minimization and privacy by design.
- Protecting Vulnerable Populations: Recruiting from groups such as children, pregnant women, prisoners, or individuals with cognitive impairments requires extra safeguards to prevent coercion or undue influence. This often involves obtaining consent from a legally authorized representative (e.g., parental consent) in addition to the participant’s assent (their affirmative agreement), and requires heightened scrutiny from IRBs.
Conclusion
The statistic is stark: 76% of trials fail due to poor recruitment. This means stalled research, lost investment, and patients waiting for life-changing treatments. The key takeaway is that the old ways of recruiting are broken. We can no longer design trials in a vacuum and treat recruitment as an afterthought.
The path forward requires a new approach:
- Diagnose the funnel: Distinguish between recruitment and enrollment to pinpoint and fix leaks.
- Leverage technology: Use AI and Real-World Data to overcome barriers like complex protocols and strict criteria, finding the right patients faster.
- Put patients first: Design trials that minimize burden, communicate clearly, and actively recruit diverse populations to build trust.
Patient-centricity is what truly moves the needle. This extends to retention, where keeping participants engaged protects data integrity. All of this must happen within the ethical guardrails of informed consent and data privacy.
At Lifebit, our federated AI platform directly addresses the bottleneck of accessing diverse patient data while ensuring privacy. Our technology lets you identify eligible patients across distributed datasets (EHRs, genomics, claims) without moving sensitive information. This leads to faster feasibility, precise targeting, and on-time enrollment.
The future of clinical research combines cutting-edge technology with human-centered design. We can help you turn patient recruitment in clinical trials from your biggest challenge into a competitive advantage.

