Real-World Data Jobs Where the Opportunities Are
Real-World Data Jobs: 5 Roles Helping You Turn Health Data Chaos Into Patient Impact
Real world data jobs are exploding across pharma, biotech, and healthcare as organizations race to harness patient information from electronic health records, claims databases, and genomic datasets. If you’re looking to break into this field, here are the top opportunities right now:
Top Real-World Data Job Roles:
- Real-World Evidence (RWE) Data Scientist – Analyze patient data to generate clinical insights and support drug development
- RWD Data Engineer – Build pipelines that harmonize fragmented health data from multiple sources
- Health Economics and Outcomes Research (HEOR) Manager – Demonstrate treatment value to payers and regulators
- Clinical/RWD Epidemiologist – Design observational studies and analyze population health patterns
- RWD Product Manager – Lead strategy and translate business needs into technical solutions
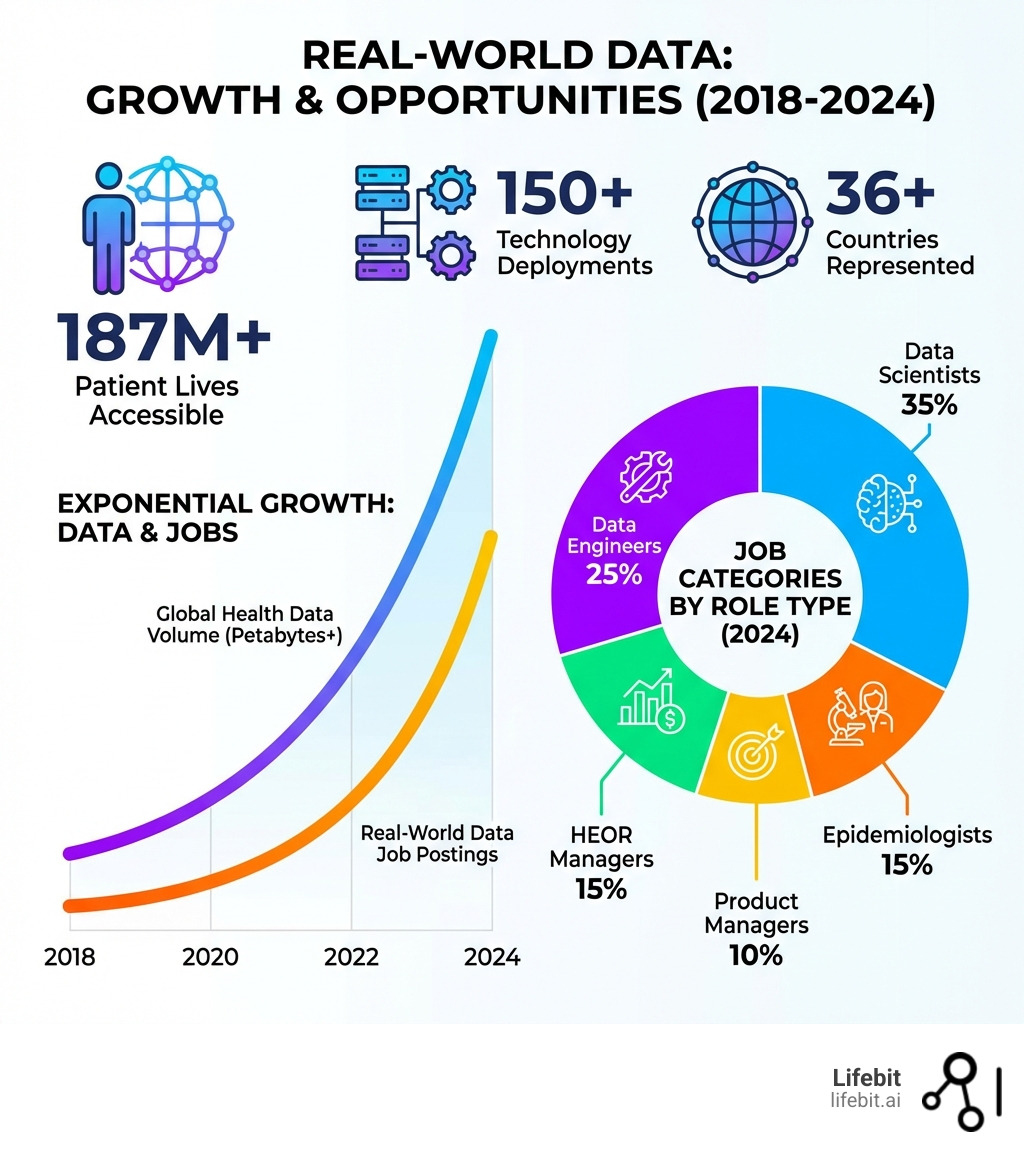
The numbers tell the story. Over 187 million patient lives are now accessible through modern data platforms, with more than 90% of that data coming from outside the US. Companies like Johnson & Johnson have 52 open data science positions right now. The UK alone lists 181 real-world data jobs, while the US market shows over 11,000 active postings.
Why the surge? Healthcare organizations are sitting on massive volumes of fragmented, multi-modal data—electronic health records, imaging files, genomics, lab results, biomarkers—and they need skilled professionals who can transform that raw information into evidence that speeds drug findy, supports regulatory submissions, and improves patient outcomes. Real-world evidence has moved from nice-to-have to mission-critical, now supporting a growing number of FDA and EMA submissions.
But there’s a catch: most organizations struggle with slow data onboarding, poor data quality, and regulatory bottlenecks. That’s where professionals with the right mix of technical skills (Python, R, SQL, machine learning) and domain knowledge (pharmacoepidemiology, clinical trials, regulatory frameworks) become invaluable.
I’m Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, a federated genomics and biomedical data platform that enables secure, compliant analysis across siloed health datasets. With over 15 years in computational biology, AI, and health-tech, I’ve seen how real world data jobs are changing from niche technical roles into high-impact careers that directly change patient lives. This guide will show you exactly where those opportunities are and how to land them.
Relevant articles related to real world data jobs:
First, What Are Real-World Data (RWD) and Real-World Evidence (RWE)?
Before we dive into the exciting real world data jobs available, let’s clarify what we mean by Real-World Data (RWD) and Real-World Evidence (RWE). RWD is the raw material – information collected from routine healthcare settings – and RWE is the valuable insight derived from analyzing that data. The crucial link between them is the change of disparate data points into actionable knowledge that can inform medical decisions, drug development, and patient care.
Real-World Data (RWD) encompasses information relating to patient health status and/or the delivery of healthcare routinely collected from a variety of sources. Real-World Evidence (RWE) is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD. Think of RWD as the ingredients, and RWE as the delicious, insightful meal prepared from them. For a deeper dive, check out our Real-World Data vs. Evidence: Ultimate Guide.
| Real-World Data (Raw Inputs) | Real-World Evidence (Analyzed Outputs) |
|---|---|
| Raw, unstructured, or structured data | Clinical insights, treatment patterns, safety signals |
| Directly collected observations | Conclusions drawn from RWD analysis |
| Foundational information | Actionable knowledge for decision-making |
| Sources: EHRs, claims, registries | Outcomes: efficacy, safety, cost-effectiveness |

What Counts as Real-World Data?
The beauty of RWD lies in its diversity and volume. It’s not just data from clinical trials, but rather the rich mix of information generated during everyday healthcare. This includes:
- Electronic Health Records (EHRs): These are digital versions of patient charts, containing diagnoses, treatments, medications, lab results, and clinical notes. They offer a comprehensive, longitudinal view of a patient’s health journey.
- Insurance claims data: Records generated from billing and reimbursement processes, providing insights into treatments, procedures, and healthcare utilization.
- Patient-generated data: Information reported directly by patients, often through surveys, mobile apps, or health diaries, capturing their symptoms, quality of life, and treatment adherence.
- Wearables and mobile devices: Data from smartwatches, fitness trackers, and other digital health tools, offering continuous monitoring of physiological parameters like heart rate, activity levels, and sleep patterns.
- Disease registries: Collections of data about patients with specific diseases, used for research, surveillance, and tracking disease progression.
For more examples and a comprehensive understanding of these diverse sources, explore our article on More on RWD sources.
How is Real-World Evidence Generated?
Generating RWE is where the magic happens – and where many real world data jobs come into play. It involves a sophisticated process of:
- Data analysis: Applying statistical methods and computational techniques to RWD to identify trends, correlations, and causal relationships.
- Statistical modeling: Building predictive models to forecast patient outcomes, identify risk factors, and simulate intervention effects.
- Generating clinical insights: Interpreting the analytical results in a clinical context to understand disease progression, treatment effectiveness, and patient safety.
- Supporting decisions: Using these insights to inform drug development strategies, regulatory submissions, treatment guidelines, and healthcare policy.
The goal is always to transform raw data into robust, reliable evidence that can withstand scientific and regulatory scrutiny. For those in pharma, understanding how to effectively generate this evidence is paramount, as detailed in Optimizing Real-World Evidence in Pharma.
Top 5 In-Demand Real-World Data Jobs You Can Land Today
The demand for professionals who can steer and extract value from Real-World Data is skyrocketing, making real world data jobs some of the most sought-after in healthcare and life sciences. These roles often offer competitive salaries, significant career growth, and the profound satisfaction of making a tangible impact on patient lives. Whether you’re a seasoned data professional or looking to pivot into a high-growth sector, the opportunities are abundant. LinkedIn alone lists over 11,000+ Real World Data Jobs in United States, with hundreds more across Europe and the UK.
Let’s explore the top 5 in-demand roles that are shaping the future of medicine.

1. Real-World Evidence (RWE) Data Scientist
The RWE Data Scientist is arguably the most pivotal role in this space. Your focus will be on leveraging advanced analytics, predictive modeling, and machine learning to generate robust insights from RWD. You’ll translate complex business questions into data analysis specifications, ensuring scientifically rigorous methods are applied. At companies like Sanofi, an RWE Data Scientist is expected to combine expertise in areas like pharmaco-epidemiology, statistical methods, Python, R, SQL, and even advanced techniques like federated learning and Bayesian statistics. You’ll be crucial in turning data into insights that improve patient outcomes and inform regulatory decisions.
- Responsibilities & Projects: Beyond just predictive modeling, you’ll be deeply involved in causal inference—a critical skill for drawing reliable conclusions from non-randomized observational data. You’ll employ methods like propensity score matching and instrumental variable analysis to mimic the conditions of a randomized controlled trial. A typical project might involve analyzing a large claims database to assess the real-world comparative effectiveness of two different drugs for hypertension, carefully controlling for dozens of potential confounding factors to provide evidence for clinical guideline updates.
- Career & Salary: This is often a terminal role for those who love hands-on data work, but it can also lead to positions like Principal Data Scientist or Head of RWE Analytics. Salaries are highly competitive, often ranging from $120,000 to $180,000+ USD depending on experience and qualifications (a PhD is often preferred).
2. RWD Data Engineer
Behind every brilliant RWE insight is a carefully prepared dataset, and that’s where the RWD Data Engineer shines. This role focuses on building, maintaining, and optimizing the data pipelines that collect, clean, transform, and store vast amounts of Real-World Data. You’ll be responsible for data harmonization – taking fragmented, multi-modal data from various sources (EHRs, claims, genomics) and making it analysis-ready. Your expertise in data modeling and architecture is critical to ensure data quality, integrity, and accessibility.
- Responsibilities & Projects: Your primary challenge is tackling the “wild” nature of RWD. You’ll design and build robust ETL (Extract, Transform, Load) pipelines and often map raw source data to a Common Data Model (CDM) like the OMOP CDM. For example, you might lead a project to integrate a hospital’s EHR system, a cancer registry, and a genomics database into a single, queryable, OMOP-compliant warehouse. This involves writing complex data transformation logic, implementing rigorous data quality checks, and ensuring the entire infrastructure is secure and scalable on a cloud platform like AWS or Azure.
- Career & Salary: A successful RWD Data Engineer can progress to Senior or Lead Data Engineer, Data Architect, or Head of Data Engineering. Given the foundational importance of this role, compensation is strong, with typical salaries in the $110,000 to $170,000 USD range, and architects earning even more.
3. Health Economics and Outcomes Research (HEOR) Manager
An HEOR Manager in the RWD space focuses on demonstrating the economic and clinical value of medical products. This role is essential for market access, pricing, and reimbursement strategies. You’ll leverage RWE to conduct cost-effectiveness analyses, burden-of-illness studies, and comparative effectiveness research, all to support payer discussions and regulatory bodies. Essentially, you’re building the evidence base that shows why a treatment is not just effective, but also a smart investment.
- Responsibilities & Projects: You are the translator between clinical evidence and economic value. For instance, an HEOR manager might use RWE to compare the total cost of care (including hospitalizations and ER visits) for patients on a new, expensive oncology drug versus the existing standard of care. The goal is to demonstrate that while the new drug’s upfront cost is higher, it reduces downstream healthcare utilization and improves quality-adjusted life years (QALYs), making it a valuable investment for a healthcare system.
- Career & Salary: This role often requires an advanced degree (MPH, PharmD, PhD) and can lead to senior director or VP-level positions in market access or health economics. With their specialized blend of scientific and economic expertise, HEOR Managers can expect salaries from $130,000 to over $200,000 USD.
4. Clinical or RWD Epidemiologist
Epidemiologists are the detectives of disease patterns. In the RWD field, a Clinical or RWD Epidemiologist designs and executes observational studies using Real-World Data to understand disease prevalence, incidence, risk factors, and treatment effectiveness in real-world populations. Your work involves rigorous study design, statistical analysis, and causal inference to draw meaningful conclusions about population health. This role often involves collaborating with data scientists to analyze large datasets and interpret findings in a clinical and public health context.
- Responsibilities & Projects: You apply classical epidemiological principles to massive, passively collected datasets. A typical project could be a post-market safety study to monitor for rare adverse events of a newly approved vaccine across millions of patient records—a scale unfeasible with traditional methods. You would be responsible for writing the study protocol, defining the cohort and outcomes, conducting the statistical analysis, and interpreting the findings in a public health context.
- Career & Salary: Career paths can lead to senior roles within pharmaceutical companies (often in safety or pharmacoepidemiology departments), regulatory agencies, or academic research institutions. Salaries for epidemiologists in the RWD space typically fall between $100,000 and $160,000 USD, with PhD-level experts commanding higher figures.
5. RWD Product Manager
The RWD Product Manager is the visionary, bridging the gap between business needs and technical solutions. Your role involves defining the strategy, roadmap, and features for RWD products or platforms. This requires deep understanding of both the scientific applications of RWD/RWE and the underlying technical complexities. You’ll lead cross-functional teams, manage stakeholders, and translate complex business requirements from researchers and clinicians into clear technical specifications for data engineers and data scientists.
- Responsibilities & Projects: You are the CEO of the RWD product. You must align the priorities of clinical researchers, data engineers, and business leaders. For example, an RWD Product Manager might have to create a business case and roadmap for developing a new NLP feature that extracts tumor characteristics from unstructured pathology reports. This involves justifying the investment, defining the feature requirements for the engineering team, and planning the go-to-market strategy for the new capability.
- Career & Salary: This is a leadership track role that can lead to Director of Product, Head of RWD Strategy, or other executive positions. As such, salaries are strong, often ranging from $140,000 to $220,000+ USD.
For more on the impact of RWD, see our article on RWD’s Impact on Price & Reimbursement.
The Essential Skills You Need to Get Hired in RWD/RWE
Landing one of these high-impact real world data jobs requires a robust skill set that spans technical prowess, domain-specific knowledge, and crucial soft skills. Building a competitive profile means mastering this trifecta, ensuring you can not only analyze data but also understand its context and communicate its implications.
Core Technical Skills
These are the foundational tools that will empower you to manipulate, analyze, and visualize Real-World Data:
- Python & R: These are the lingua franca of data science, indispensable for statistical analysis, machine learning, and data manipulation. For Python, this means fluency in libraries like Pandas for data manipulation, NumPy for numerical operations, Scikit-learn for machine learning, and Plotly or Matplotlib for visualizations. In the R ecosystem, expertise in the Tidyverse suite (including dplyr and ggplot2) for data wrangling and visualization, alongside packages for survival analysis, is highly sought after.
- SQL & NoSQL databases: You’ll be querying vast databases of patient records, claims, and other RWD sources. Strong SQL skills are non-negotiable. This isn’t just about basic
SELECTstatements; you’ll need to master complex joins, window functions, and common table expressions (CTEs) to query intricate relational databases. On the other side, familiarity with NoSQL databases like document stores (e.g., MongoDB) or graph databases (e.g., Neo4j) is a significant plus, especially for handling unstructured clinical notes or modeling patient networks. - Cloud platforms: As RWD datasets grow exponentially, they reside on cloud infrastructure. Experience with platforms like AWS, Azure, or Google Cloud is vital for scalable data storage, processing, and analytics.
- Machine Learning & Causal Inference: For extracting insights from unstructured text in EHRs (Natural Language Processing), identifying patterns in complex genomic data (Deep Learning), or building predictive models, machine learning expertise is a huge advantage. Crucially, this must be paired with a deep understanding of causal inference methods. Beyond prediction, you must be able to infer causality from observational data using techniques like Propensity Score Matching (PSM) and Inverse Probability of Treatment Weighting (IPTW) to mitigate the confounding and selection bias that are rampant in RWD.
- Data visualization tools: Being able to clearly communicate complex findings through interactive dashboards and compelling visualizations (e.g., Tableau, Power BI) is crucial for making RWE accessible to non-technical stakeholders.
At Lifebit, our federated platform is built to enable advanced RWE generation, so proficiency in these technical areas allows you to truly leverage our capabilities. See how we empower researchers to See how Lifebit enables RWE.
Critical Domain Knowledge
Technical skills are powerful, but without context, they’re like a car without a steering wheel. Domain knowledge guides your analysis and ensures your insights are clinically relevant and impactful:
- Pharmacoepidemiology: This field focuses on the study of the use and effects of drugs in large populations. Understanding its principles is fundamental for designing RWE studies. For example, when studying a new anticoagulant, this knowledge would prompt you to control for “confounding by indication”—the fact that sicker patients are more likely to receive the new drug—to avoid incorrectly attributing adverse outcomes to the drug itself when they are actually due to the underlying illness.
- Clinical trial process: Even though RWD is distinct from traditional clinical trials, knowledge of trial design, endpoints, and regulatory requirements helps bridge the gap and allows RWE to complement and inform clinical development.
- Regulatory frameworks (FDA/EMA): Familiarity with how regulatory bodies like the FDA in the US and the EMA in Europe view and use RWE is critical for ensuring your analyses meet submission standards. This includes being familiar with the FDA’s framework for RWE, the 21st Century Cures Act, and the EMA’s initiatives like the DARWIN EU® network. Understanding how to prepare a study protocol and statistical analysis plan (SAP) that will be acceptable to these bodies is a make-or-break skill. Learn more about the US Regulatory Guidance on RWD.
- Therapeutic areas (Oncology, Immunology, Neurology): Specializing in specific disease areas allows for deeper understanding of the clinical questions, data nuances, and patient populations. Many companies, including J&J and Sanofi, emphasize expertise in areas like oncology, immunology, and rare diseases.
Essential Soft Skills for Real-World Data Jobs
Beyond the technical and domain-specific, certain soft skills are indispensable for success in real world data jobs:
- Problem-solving: RWD is often messy and complex. You need to be adept at identifying challenges, breaking them down, and devising creative solutions.
- Communication: You’ll be working with diverse teams – clinicians, statisticians, business leaders, and regulators. The ability to clearly articulate complex technical concepts and present findings in an understandable way is paramount. Sanofi’s job descriptions frequently highlight the need for clear communication.
- Collaboration: RWD projects are rarely solo endeavors. Working effectively in multidisciplinary teams, sharing knowledge, and leveraging collective expertise is key, as emphasized by J&J’s focus on “smart collaboration” and “dream teams.”
- Business acumen: Understanding the strategic objectives of your organization or client (e.g., drug development, market access, patient care improvement) allows you to align your analytical efforts with real-world impact.
- Growth mindset: The RWD field is constantly evolving. A willingness to continuously learn new technologies, methodologies, and therapeutic areas is vital for long-term career growth.
How RWD is Revolutionizing Drug Development and Patient Care
Real-World Data and Evidence are not just buzzwords; they are powerful catalysts changing the entire healthcare ecosystem. From accelerating the findy of new therapies to tailoring treatments for individual patients, RWD is making healthcare smarter, faster, and more effective.
Accelerating Drug Findy and Clinical Trials
The traditional drug development pipeline is notoriously long, costly, and often inefficient. RWD offers a powerful antidote, fundamentally changing how we approach clinical research:
- Smarter trial design: RWD can inform patient selection criteria, identify optimal study sites, and even predict potential challenges, leading to more efficient and targeted clinical trials.
- Faster patient recruitment: By analyzing EHRs and claims data, researchers can quickly identify eligible patients for trials, dramatically speeding up the recruitment process. Lifebit’s federated approach, for instance, has enabled rapid, compliant patient matching, accelerating cohort recruitment by 60% across 100 institutions in some cases.
- External control arms: For rare diseases or situations where traditional placebo groups are unethical, RWD can create synthetic control arms, reducing the need for large patient cohorts and accelerating regulatory approval.
- Reducing development costs: By optimizing trial design and recruitment, RWD can significantly cut down the time and resources required for drug development.
The benefits are clear: RWD is a game-changer for clinical research. Dive deeper into these advantages with our article on Benefits of RWD in Clinical Research.
Powering Personalized Medicine
The vision of personalized medicine – delivering the right treatment to the right patient at the right time – is becoming a reality thanks to RWD.
- Targeted therapies: By analyzing RWD, we can identify patient subgroups that respond best to specific treatments, leading to more effective and less toxic therapies. This is particularly impactful in areas like oncology, where RWD helps us understand how different patient profiles react to various cancer treatments.
- Understanding disease progression: Longitudinal RWD provides an unparalleled view of how diseases evolve over time, allowing for earlier intervention and more precise management strategies. J&J, for example, uses data science to predict Alzheimer’s disease progression.
- Improving patient outcomes: Personalized medicine driven by RWD leads to better treatment decisions, fewer adverse events, and a higher quality of life for patients. Our article on RWD for Clinical Evidence in Oncology highlights how RWD helps generate critical clinical evidence in complex disease areas.
Informing Regulatory and Market Access Decisions
RWD is increasingly vital for gaining approval for new drugs and ensuring they reach the patients who need them.
- Supporting FDA/EMA submissions: Regulatory bodies are recognizing the value of RWE. RWD can provide supplementary evidence for drug efficacy and safety, particularly for post-market surveillance or expanding indications, with a growing number of regulatory submissions now incorporating real-world evidence.
- Demonstrating treatment value: For market access and reimbursement, RWD helps pharmaceutical companies demonstrate the real-world effectiveness and cost-effectiveness of their products to payers and healthcare systems. This includes generating robust datasets to support payer discussions and Health Technology Assessment (HTA) evidence.
- Post-market surveillance: After a drug is approved, RWD continues to play a critical role in monitoring its safety and effectiveness in the broader patient population, identifying rare side effects or long-term benefits that might not have been apparent in controlled clinical trials.
The Future of the Field: Challenges and Opportunities in Real-World Data Jobs
The landscape of real world data jobs is dynamic, shaped by rapid technological advancements and evolving ethical considerations. As we look ahead, the integration of Artificial Intelligence and Machine Learning, coupled with the critical need for robust data privacy frameworks, defines both the challenges and the immense opportunities for career growth.
The Rise of AI, ML, and Federated Learning
Artificial Intelligence and Machine Learning are no longer futuristic concepts; they are integral to extracting meaningful insights from the explosion of RWD.
- AI for predictive insights: AI models can analyze vast datasets to predict disease outbreaks, patient responses to therapies, or identify individuals at high risk for certain conditions. These models are increasingly used for complex tasks like survival analysis and propensity score matching.
- NLP on clinical notes: Much of the valuable information in EHRs is buried in unstructured text. Natural Language Processing (NLP) allows us to automatically extract diagnoses, symptoms, treatments, and outcomes from these notes, opening up previously inaccessible data for analysis. J&J uses NLP applications to make surgery safer and transform how rare diseases are diagnosed.
- Federated technology for secure data access: With data often residing in siloed systems across different institutions and countries, federated learning emerges as a powerful solution. This approach allows AI models to be trained on distributed datasets without the data ever leaving its source, addressing privacy concerns while still enabling large-scale analysis. Our own federated AI platform at Lifebit embodies this, enabling secure, real-time access and analysis across global biomedical data.
Despite these advancements, working with RWD presents unique challenges, as explored in Challenges of Using RWD in Research.
Navigating Data Privacy and Ethical Problems
Working with sensitive patient data comes with immense responsibility. Ethical considerations and data privacy are paramount in all real world data jobs.
- Data quality and bias: RWD is often collected for clinical care, not research, leading to inconsistencies and potential biases. Professionals must be skilled in data cleaning, validation, and bias mitigation techniques.
- GDPR compliance: For those working in Europe and the UK, adhering to strict regulations like the General Data Protection Regulation (GDPR) is non-negotiable. This involves careful anonymization, consent management, and data governance.
- Anonymization techniques: Implementing robust anonymization and de-identification methods is crucial to protect patient privacy while still allowing for valuable research.
- Trusted Research Environments (TREs): These secure, controlled environments provide a safe space for researchers to access and analyze sensitive RWD without compromising patient confidentiality. Lifebit’s platform includes a Trusted Research Environment designed for this very purpose, ensuring compliant research within hybrid data ecosystems.
Understanding your rights and the policies governing data is fundamental. For more information, you can always Learn about data privacy rights.
Your Career Growth and Future Outlook
The outlook for real world data jobs is exceptionally bright. The demand for talented professionals far outstrips supply, creating a robust job market with significant opportunities for growth.
- Growing demand for talent: As healthcare systems increasingly rely on RWE for decision-making, the need for skilled data scientists, engineers, epidemiologists, and product managers will only intensify. LinkedIn shows a vibrant market, with 827 Real World Evidence jobs in New York and 181 Real World Data jobs in UK alone.
- Expansion into new therapeutic areas: While oncology and rare diseases have been early adopters, RWD is expanding its influence across cardiology, neurology, immunology, and beyond, opening up diverse specialization paths.
- Global job market: The need for RWD expertise is global. With companies like Lifebit operating across 5 continents, and data access spanning dozens of countries, opportunities span the USA, UK, Europe, Canada, Singapore, and Israel, offering exciting international career prospects.
Conclusion
The field of real world data jobs is not just a burgeoning sector; it’s a critical frontier in modern healthcare. We’ve seen how RWD and RWE are changing everything from drug findy and clinical trials to personalized medicine and regulatory decisions. This is a field where combining data science prowess with a deep understanding of health outcomes directly leads to saving lives and improving patient care.
The opportunities are immense, with roles like RWE Data Scientists, RWD Data Engineers, HEOR Managers, Clinical Epidemiologists, and RWD Product Managers leading the charge. To thrive in these roles, you’ll need a blend of core technical skills (Python, R, SQL, ML), critical domain knowledge (pharmacoepidemiology, regulatory frameworks), and essential soft skills (communication, collaboration).
As the healthcare industry continues its digital change, driven by the power of AI, ML, and federated learning, the demand for professionals in this space will only grow. At Lifebit, we are proud to be at the forefront of this revolution, providing the federated technology that enables secure, compliant, and transformative research. If you’re ready to make a profound impact and shape the future of medicine, the time to explore real world data jobs is now.
Take the next step in innovating healthcare with federated data strategies

