Health’s New Horizon: A Guide to Healthcare Innovation Platforms

Cut R&D Timelines & Costs: The Platform Powering 20+ FDA/EMA Submissions
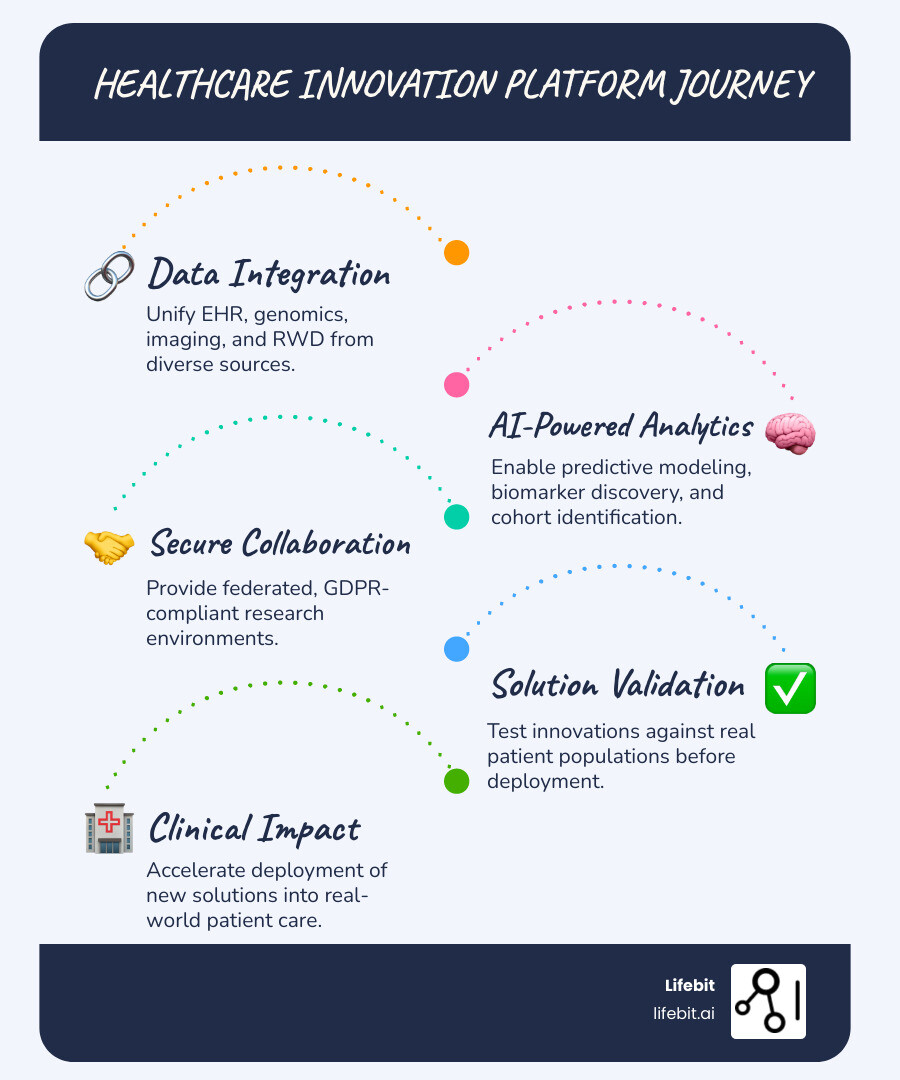
A healthcare innovation platform is a digital infrastructure that connects data, developers, and providers to accelerate the development, validation, and deployment of new healthcare solutions. These platforms break down data silos, enable secure collaboration, and use AI to transform clinical information into actionable insights—ultimately shortening the path from research concept to real-world patient care.
Key capabilities of a healthcare innovation platform:
- Data integration – Unifies EHR, genomics, imaging, and real-world data from diverse sources
- AI-powered analytics – Enables predictive modeling, biomarker findy, and cohort identification
- Secure collaboration – Provides federated, GDPR-compliant research environments
- Solution validation – Tests innovations against real patient populations before deployment
- Regulatory support – Generates evidence for FDA/EMA submissions and market access
Healthcare is fragmented. Researchers struggle to access the data they need. Clinicians wait years for innovations to reach their workflows. Costs rise while outcomes stagnate. The current system wasn’t built for the speed or complexity of modern medicine.
Healthcare innovation platforms address these bottlenecks by creating an ecosystem where data, digital solutions, and expertise flow seamlessly between innovators and care teams. With access to 187M+ patients across 36+ countries, these platforms enable real-world evidence generation at unprecedented scale. They’ve already supported over 20 FDA/EMA submissions and helped healthcare organizations reduce development timelines while improving patient outcomes.
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve spent over 15 years building genomics and healthcare innovation platforms that power federated data analysis for global pharmaceutical companies and public health institutions. My work focuses on making precision medicine scalable by breaking down the technical and regulatory barriers that slow innovation.
The shift from isolated research efforts to connected innovation ecosystems represents a fundamental change in how we develop and deliver healthcare. Below, you’ll see how these platforms turn fragmented data into coordinated action.
Healthcare innovation platform terms simplified:
Your Data Is a Mess. Here’s the 5-Part Blueprint to Unify It.
A modern healthcare innovation platform serves as the central nervous system for advancing medical science and patient care. Its core concept is to provide a unified, secure environment where diverse stakeholders can access, analyze, and collaborate on vast amounts of health data. This infrastructure is designed to break down the traditional data silos that have long hampered progress, accelerating research and development by connecting innovators, clinicians, and health systems.
At its heart, such a platform aims to address pressing healthcare challenges, from rising costs and workforce shortages to inefficiencies and the growing complexity of patient care. It reimagines healthcare as an ecosystem where data, digital solutions, and expertise flow seamlessly, changing data into findies and findies into better care.

Key Components of a healthcare innovation platform
To achieve this ambitious goal, a robust healthcare innovation platform typically comprises several critical components:
- Data Integration Layer: This is the foundation, responsible for ingesting, harmonizing, and making diverse data types accessible. It acts as a universal translator for health information. However, this process is fraught with technical challenges. Healthcare data is notoriously heterogeneous, existing in countless proprietary formats and siloed systems. To overcome this, advanced platforms employ a sophisticated ETL (Extract, Transform, Load) process coupled with standardized data models. For example, mapping source data to a Common Data Model (CDM) like the Observational Medical Outcomes Partnership (OMOP) CDM allows for systematic analysis across disparate databases. Furthermore, the adoption of interoperability standards like Fast Healthcare Interoperability Resources (FHIR) is critical, enabling data to be exchanged between different software systems in a consistent, API-driven manner.
- Analytics and AI Workbench: Equipped with advanced AI/ML capabilities, this component provides tools for researchers and developers to explore data, build models, and generate insights. Platforms like ours include sophisticated AI-guided tools and predictive analytics to accelerate findy.
- Collaboration Tools: Secure environments for multiple parties—from academic institutions to health tech companies—to work together on projects, share findings, and co-develop solutions.
- Governance and Security Framework: Crucial for maintaining trust and compliance, this involves robust privacy-preserving technologies, access controls, and audit trails to ensure data is handled ethically and legally. Beyond standard encryption and access controls, leading platforms incorporate advanced Privacy-Enhancing Technologies (PETs). For instance, differential privacy involves adding statistical noise to query results, making it impossible to re-identify any single individual while preserving the accuracy of large-scale analyses. Secure multi-party computation (SMPC) allows multiple parties to jointly compute a function over their inputs without revealing those inputs to each other. This technical framework is complemented by rigorous ethical and administrative governance, including oversight from Institutional Review Boards (IRBs) and data access committees, ensuring every research query is scientifically valid and ethically sound.
- Solution Deployment Module: A pathway for validated innovations to be integrated into clinical workflows, ensuring that research translates into real-world impact and improved patient outcomes.
The Data Foundation: Integrating Diverse Health Information
The power of a healthcare innovation platform lies in its ability to integrate and leverage a wide array of health data. This isn’t just about collecting data; it’s about harmonizing it into a usable format, often from disparate sources and in various forms. Our platform, for instance, focuses on open uping the full potential of global health data through advanced technology and predictive analytics.
The types of data integrated are extensive and multimodal:
- Structured Data: This includes coded information like diagnoses (ICD codes), lab results, medication lists, and administrative data such as claims and safety/quality reports.
- Unstructured Data: Clinical notes, pathology reports, and other free-text entries from Electronic Health Records (EHRs) provide rich context often missed by structured data alone.
- Electronic Health Records (EHR): The bedrock of clinical data, offering longitudinal patient histories.
- Genomics and Multi-omics Data: Information from DNA sequencing, proteomics, metabolomics, and other ‘omics’ fields, crucial for precision medicine and biomarker findy.
- Medical Imaging: X-rays, MRIs, CT scans, and other visual data, increasingly analyzed with AI to aid diagnosis and treatment planning.
- Waveform Data: Continuous physiological measurements from medical devices, such as ECGs, EEGs, and vital sign monitors.
- Real-World Data (RWD): Data relating to patient health status and/or the delivery of healthcare routinely collected from a variety of sources. This includes data from electronic medical records, claims and billing data, product and disease registries, and even data from personal devices and health applications.
With access to vast datasets, such as the 187M+ patients accessible through leading platforms, and with over 90% of that data often originating from outside the US, the scale of insight generation is truly unprecedented. This global reach, spanning 36+ countries, allows for comprehensive research and validation across diverse populations, a critical factor for developing universally applicable solutions.
Ensuring Security and Privacy in a Data-Rich World
In healthcare, data privacy and security are paramount. Healthcare innovation platforms must steer complex regulatory landscapes like GDPR and HIPAA while enabling necessary data access for research and development. This is where advanced architectural approaches come into play.
- Federated Architecture: This allows data to remain at its source, within the control of the data custodian (e.g., a hospital or research institute), while researchers can run analyses remotely. Our federated AI platform enables secure, real-time access to global biomedical and multi-omic data without centralizing sensitive information. This preserves privacy while still allowing for large-scale analysis.
- De-identification and Anonymization: Techniques used to remove or mask personally identifiable information from datasets, rendering them safe for research without compromising patient privacy.
- GDPR and HIPAA Compliance: Platforms are built with these stringent regulations in mind, ensuring legal and ethical data handling across different jurisdictions, including the UK, USA, and Europe.
- Trusted Research Environments (TREs): These secure, controlled environments provide a safe space for researchers to access and analyze sensitive data. TREs, like those we incorporate, are designed with strict access controls, audit trails, and data governance policies to prevent unauthorized access or data breaches.
The NHS, for example, provides services to support innovators in understanding the regulatory landscape and ensuring their solutions meet essential standards before adoption. Learn how the NHS supports innovators to get solutions adopted safely. This emphasis on secure, compliant data access is fundamental to building trust and fostering responsible innovation within the healthcare sector.
Stop Guessing: How AI Turns Raw Health Data into FDA-Ready Evidence.
Artificial intelligence (AI) and machine learning (ML) are not just buzzwords in healthcare; they are the core engine driving innovation within these platforms. They transform raw, complex health data into actionable insights, accelerating findy and enabling more precise, personalized care.
The role of AI and machine learning in a healthcare innovation platform is multifaceted:
- Predictive Analytics: AI models can predict disease progression, patient response to treatments, or even outbreaks, allowing for proactive interventions.
- Diagnostic Support: AI can analyze imaging data or EHRs to assist clinicians in faster, more accurate diagnoses.
- Drug Findy and Development: ML algorithms can identify potential drug targets, predict molecular interactions, and optimize clinical trial design, significantly shortening development timelines.
- Foundation Models in Medicine: These large, pre-trained AI models are emerging as a paradigm shift, capable of understanding and generating medical text, images, and other data types, promising to revolutionize medical AI.

From Raw Data to Actionable Insights
One of the greatest challenges in healthcare research is making sense of disparate data. A healthcare innovation platform addresses this through:
- Data Harmonization: Our platform, for instance, excels at ingesting, harmonizing, changing, and enriching multi-modal data. This ensures that data from different sources and formats can be consistently interpreted and analyzed.
- AI-powered Analytics: With harmonized data, AI algorithms can perform advanced analytics to uncover hidden patterns. This includes identifying patient cohorts for clinical trials, finding new biomarkers for disease, and analyzing standard of care patterns. Our platform provides AI-driven safety surveillance and real-time insights, essential for pharmacovigilance.
- Real-World Evidence (RWE) Generation: By analyzing real-world data at scale, platforms can generate robust evidence to support regulatory submissions, market access, and post-launch activities. This is critical for demonstrating the effectiveness and safety of new interventions in diverse patient populations.
- Biomarker Findy and Patient Cohort Identification: AI can rapidly process vast genomic and clinical datasets to pinpoint biomarkers indicative of disease or treatment response, and to identify specific patient groups that would benefit most from a particular therapy.
This rigorous analytical capability is why such platforms have been instrumental in supporting over 20 FDA / EMA submissions, helping life sciences companies and public health agencies bring innovations to patients faster and with greater confidence. A critical component of this process, especially for clinical adoption, is AI Explainability (XAI). Clinicians are unlikely to trust a ‘black box’ algorithm’s recommendation without understanding its reasoning. Therefore, modern platforms are integrating XAI techniques that provide insights into how a model arrived at a conclusion. Methods like SHAP (SHapley Additive exPlanations) can highlight which specific data points (e.g., a particular lab value or a phrase in a clinical note) most influenced a prediction. This transparency builds trust, aids in debugging models, and can even lead to new clinical discoveries by revealing unexpected correlations.
Validating and Deploying New Digital Health Solutions
It’s not enough to simply develop innovative solutions; they must be rigorously validated and seamlessly deployed into clinical practice. A healthcare innovation platform provides the necessary infrastructure and processes for this critical journey.
The validation-to-deployment pathway typically involves:
- Hypothesis Testing with Real-World Data: Innovators can test their ideas and hypotheses against real patient populations and vast datasets. Access to curated, de-identified clinical data enables early signal detection and feasibility analysis, reducing uncertainty.
- Rigorous Qualification for Safety and Fairness: AI-enabled solutions, especially, undergo a rigorous qualification process to ensure they are safe, fair, effective, and provide real-world value. This involves testing for accuracy, clinical utility, and critically, auditing for algorithmic bias. An AI model trained predominantly on data from one demographic group may perform poorly or unfairly when applied to others. For example, a skin cancer detection algorithm trained primarily on light-skinned individuals might fail to accurately identify melanomas in patients with darker skin, exacerbating existing health disparities. Healthcare innovation platforms facilitate this crucial step by providing access to diverse, representative datasets, allowing developers to test and mitigate bias across different races, ethnicities, genders, and socioeconomic groups before a solution is deployed.
- Integration into Clinical Workflows: Once validated, digital health tools and AI solutions are integrated directly into clinical and administrative workflows. This could mean embedding an AI diagnostic tool into an EHR system or providing clinicians with vetted digital health apps. This integration leads to improved diagnoses, streamlined care delivery, and reduced staff burden.
- Post-market Surveillance and Monitoring: The platform also supports ongoing monitoring of deployed solutions in real-world settings, gathering data on their performance, safety, and effectiveness to ensure continuous improvement and long-term impact.
Your Innovation Is Stuck. Here’s the Blueprint for FDA Approval and Market Access.
The true power of a healthcare innovation platform lies in its ability to foster collaboration. Healthcare innovation is rarely an isolated endeavor; it thrives in an ecosystem where diverse perspectives and expertise converge. These platforms are designed to connect innovators, clinicians, health systems, researchers, and life sciences companies, creating a powerful collective for progress.
This ecosystem approach allows for a seamless flow of data, digital solutions, and expertise. Leading platforms bring together technology developers, providers, and researchers, accelerating the journey from concept to clinical impact. Whether it’s a major academic institution partnering with venture builders to create new companies, or global organizations leveraging staff and member states, collaboration is key.
Accelerating the Journey from Concept to Clinical Impact
The path from a brilliant idea to a deployed solution in healthcare can be long and arduous. Healthcare innovation platforms aim to drastically shorten this journey by:
- Shortening Timelines and Reducing Development Cycles: By providing immediate access to data, computational resources, and collaborative tools, platforms cut down the time spent on data acquisition, preparation, and infrastructure setup. This means faster experimentation and iteration.
- Rapid Idea Validation: Innovators can prototype solutions in a secure sandbox environment and quickly validate them against real-world data, allowing for early feedback and adjustments before significant investment. Consider a hypothetical startup, ‘NeuroDetect,’ aiming to create an AI tool that predicts early-onset Alzheimer’s disease from retinal scans and cognitive test scores. Instead of spending years negotiating individual data access agreements, NeuroDetect uses a healthcare innovation platform. Within a secure, federated environment, they access a multi-modal dataset from several international health systems. They train their model without ever moving or seeing the raw patient data. The platform’s tools allow them to test the model’s performance across different ethnic groups, identifying and correcting for bias early on. Clinicians within the platform’s network can then review the de-identified results and provide feedback, helping NeuroDetect refine the tool’s clinical utility long before embarking on a formal trial.
- Real-World Testing: Solutions can be tested and fine-tuned in actual clinical settings, ensuring they are not just theoretically sound but practically effective and operationally scalable. Cedars-Sinai, for example, acts as a first large-scale customer for new companies, validating solutions in their clinical environment.
Global initiatives, such as the WHO’s LEAD Innovation Challenge, exemplify this collaborative spirit, engaging staff, member states, and partners to generate and develop solutions for public health challenges. The WHO is fostering global health collaboration through its innovation initiatives. Such programs demonstrate how platforms can accelerate the development of solutions for critical global health needs.
Supporting Commercialization and Scaling New Solutions
Bringing a new healthcare innovation to market involves more than just scientific breakthroughs; it requires navigating complex business and regulatory landscapes. Healthcare innovation platforms provide crucial support for commercialization and scaling:
- Market Access Strategies: Platforms help innovators understand the market needs, identify target populations, and develop strategies for successful adoption.
- Navigating Regulatory Pathways: Generating the necessary evidence for regulatory approval (e.g., FDA, EMA) is a core function. Platforms facilitate the collection and analysis of data required for these submissions, ensuring compliance and accelerating the approval process. This evidence generation is nuanced. For a regulatory body like the FDA, the primary focus is on safety and clinical efficacy. The platform helps generate data for premarket submissions, 510(k) clearance, or De Novo classification. However, for payers and reimbursement bodies (e.g., CMS in the U.S. or NICE in the U.K.), the focus shifts to economic value and cost-effectiveness. Innovators must demonstrate that their solution not only works but also saves the health system money, reduces hospital readmissions, or improves quality-of-life-adjusted years. The platform’s ability to analyze real-world cost and utilization data alongside clinical outcomes is invaluable for building this health economics and outcomes research (HEOR) case, which is essential for securing favorable reimbursement decisions and achieving widespread market adoption.
- Understanding Reimbursement Processes: For solutions to be widely adopted, they must be reimbursable. Platforms can provide insights into current reimbursement models and support the generation of evidence needed to demonstrate economic value. The NHS Innovation Service, for instance, guides innovators through these processes in the UK.
- Access to Global Provider Networks: By connecting innovators with a global network of providers, platforms offer scalable pathways to adoption, enabling solutions to reach a wider patient base and deliver real-world impact across leading health systems. Our platform supports biopharma and healthcare organizations globally, fostering a vast ecosystem for medical breakthroughs.
- Scaling Adoption Across Health Systems: Platforms facilitate the deployment of solutions across multiple health systems, ensuring that innovations are not confined to a single institution but can benefit patients on a larger scale.
The End of Reactive Medicine: How to Predict Disease and Personalize Treatment
The tangible benefits of healthcare innovation platforms for providers are significant and immediate. These platforms are designed to address the daily challenges faced by clinicians and healthcare organizations, leading to a measurable improvement in care delivery and operational efficiency.
- Improved Diagnosis: AI-powered tools provide clinicians with more accurate and faster diagnostic capabilities, particularly in complex cases or rare diseases. The ability to access diverse patient data through a network can lead to better care, as seen in cases where patients with rare diagnoses receive improved support through network consultations.
- Personalized Medicine: By leveraging genomic and real-world clinical data, platforms enable the development and application of personalized therapies custom to an individual patient’s genetic makeup and unique health profile, improving treatment efficacy and reducing adverse effects.
- Streamlined Workflows and Reduced Staff Burden: Digital health tools and AI solutions integrated into clinical workflows automate routine tasks, reduce administrative overhead, and free up clinicians to focus more on patient care. This can alleviate the pressure on healthcare staff, a critical benefit given ongoing workforce shortages.
- Better Patient Outcomes: The goal of all these innovations is to improve patient health. From more precise treatments to earlier disease detection and more efficient care delivery, platforms contribute directly to improved patient outcomes and a higher quality of life.
Empowering Patients as Research Partners
Beyond improving provider workflows, these platforms are beginning to democratize the research process by empowering patients. Historically, patients were passive subjects in clinical studies. The new paradigm positions them as active partners. Platforms can provide secure patient portals where individuals can access their own health data, receive information about relevant clinical trials, and provide electronic consent to share their de-identified data for specific research projects. This patient-centric approach not only accelerates trial recruitment but also ensures research is more aligned with patient needs and priorities. Initiatives like patient-reported outcome measures (PROMs) can be integrated directly, capturing the patient’s perspective on treatment effectiveness and quality of life, providing a more holistic view of a therapy’s impact.
The Future Role of the healthcare innovation platform
The future of healthcare is inextricably linked with the evolution of the healthcare innovation platform. We anticipate these platforms will continue to drive a fundamental shift in how healthcare is delivered.
- Changing Healthcare Delivery: The move towards more virtual care, remote monitoring, and personalized interventions will be increasingly powered by these platforms. The rapid response of platforms like Anthem’s Sydney Care app during the COVID-19 pandemic, providing over 40,000 assessments and 260,000 downloads, demonstrated the agility and impact of digital-first healthcare strategies.
- Powering Population Health Initiatives: Platforms will play a crucial role in enabling national healthcare initiatives by providing the data and analytics needed to understand population health trends, predict public health crises, and implement targeted interventions. The WHO’s focus on “AI for all” in public health underscores this global imperative.
- The Shift to Proactive and Predictive Care: Healthcare will become less reactive and more proactive. AI and machine learning will allow for earlier identification of health risks, enabling preventive measures and personalized wellness plans that keep people healthy rather than just treating illness. For example, instead of waiting for a patient to present with symptoms of heart failure, a platform can continuously analyze a combination of data streams: EHR data (e.g., rising blood pressure, new prescriptions), real-world data from a patient’s smartwatch (e.g., decreased activity levels, irregular heart rhythms), and genomic risk scores. An AI model could flag this individual as being at high risk for a cardiac event in the next six months. This triggers a proactive intervention: an automated alert to their care manager, enrollment in a digital cardiac rehab program via a smartphone app, and a telehealth consultation to adjust medication. This shift from reactive treatment to predictive prevention is the ultimate promise of a fully integrated healthcare innovation platform.
- Continued Integration of Advanced AI: As AI technologies mature, we’ll see even more sophisticated models, including foundation models, being applied across all aspects of healthcare, from basic research to complex clinical decision support.
- Global Data Networks: The expansion of global data networks will be paramount. With platforms already engaging in 36+ countries and with 150+ data partnerships, the ability to access and analyze diverse patient populations across continents will accelerate findies that are globally relevant and equitable. This comprehensive reach ensures that innovations are not limited by geographical boundaries but can benefit humanity worldwide.
Your Next Breakthrough Is Waiting. Here’s How to Find It.
We stand at the cusp of a profound change in healthcare, driven by the rise of the healthcare innovation platform. These sophisticated digital infrastructures are dismantling traditional barriers, unifying fragmented data, and using the immense power of artificial intelligence to accelerate findy and deliver more effective, personalized care.
We’ve seen how these platforms define a new paradigm for medical advancement, integrating diverse data types from EHRs to multi-omics, and ensuring rigorous security and privacy through federated architectures and Trusted Research Environments. They act as the engine room for AI and machine learning, converting raw data into actionable insights that fuel drug development, enable precision medicine, and support critical regulatory submissions.
Furthermore, these platforms foster a collaborative ecosystem, connecting innovators, clinicians, and health systems to accelerate the journey from concept to clinical impact, while also providing the necessary support for commercialization and scaling new solutions globally. The real-world benefits are clear: improved diagnosis, personalized treatments, streamlined workflows, reduced staff burden, and ultimately, better patient outcomes.
The future of healthcare is undeniably data-driven and collaborative. Platforms like ours are not just facilitating innovation; they are essential for building this future. By providing secure, real-time access to global biomedical and multi-omic data, with built-in capabilities for harmonization, advanced AI/ML analytics, and federated governance, we are powering large-scale, compliant research and pharmacovigilance across biopharma, governments, and public health agencies.
The era of isolated research is giving way to an interconnected, intelligent ecosystem. Join us as we explore the immense potential of this new horizon.
Explore our federated platform to see how you can accelerate your innovation journey.

