The Human Element: What Does a Drug Trial Really Mean?

Understanding What a Clinical Trial Really Means
Clinical trial means a research study where human volunteers participate to test whether new medical treatments, drugs, devices, or interventions are safe and effective. While the term is often used in medical journals and news reports, its implications for human health are profound. At its core, a clinical trial is the final, most rigorous step in a long journey of scientific discovery that begins in a laboratory.
Historically, the concept of a clinical trial has evolved from simple observations to the complex, multi-billion dollar industry it is today. One of the earliest recorded trials was conducted by James Lind in 1747 to treat scurvy among sailors. Today, the process is governed by international standards of Good Clinical Practice (GCP) to ensure that the data generated is credible and that the rights of participants are protected.
A clinical trial is defined by several core characteristics:
- A prospective study: Unlike retrospective studies that look at past data, clinical trials are planned in advance to test specific health interventions on human participants.
- The Gold Standard for Evidence: They are the only way to determine with scientific certainty if new treatments work and are safe for the general population.
- Regulatory Requirement: They are mandatory before the FDA (U.S. Food and Drug Administration) or EMA (European Medicines Agency) grants approval for new drugs or medical devices.
- Phased Progression: They are conducted in sequential phases (1-4), each designed to answer specific research questions while minimizing risk.
- Ethical Governance: Every trial is governed by strict ethical protocols, Institutional Review Board (IRB) oversight, and informed consent requirements.
Key facts and statistics regarding the trial landscape:
- Phase 1 trials involve 20-100 people, primarily testing safety, metabolism, and dosage levels.
- Phase 3 trials involve 300-3,000 people to confirm effectiveness and monitor side effects in a larger population.
- Success Rates: Only about 10% of drugs that enter human clinical trials eventually receive FDA approval, highlighting the difficulty of medical innovation.
- Timeline: The complete process from initial laboratory discovery to final market approval typically takes between 7 and 15 years.
Every medical breakthrough you’ve heard about—from life-saving cancer immunotherapies to the rapid development of mRNA vaccines and advanced surgical robotics—exists because volunteers agreed to participate in clinical research. Without clinical trials, there would be no proven treatments, no FDA-approved medications, and no way to know if a promising finding in the lab actually helps real patients. Despite their importance, participation remains a challenge; fewer than 5% of adults with cancer will ever participate in a drug trial, and the elderly make up just 14% of trial participants despite consuming over one-third of all medications. This gap in participation can lead to a lack of data on how treatments affect diverse populations.
As Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve spent over 15 years building platforms that accelerate drug discovery and clinical research through secure, federated data analysis—work that depends entirely on understanding what clinical trial means for patients, researchers, and the future of precision medicine. My team and I support pharmaceutical organizations and public health institutions in changing how clinical data powers evidence generation across compliant, distributed environments. By making data more accessible and secure, we aim to shorten the time it takes for a trial to move from the lab to the patient.
What a Clinical Trial Means for Modern Medicine
When we talk about what a clinical trial means, we are looking at the bridge between a scientific theory and a life-saving reality. It is the experimental step of the scientific method applied to human health. Researchers use these studies to answer specific, measurable questions: Does this new drug lower blood pressure more effectively than the current standard? Is this surgical robot more precise than a human hand? Can a specific change in diet prevent cognitive decline in high-risk populations?
Broadly, clinical research is categorized into two main buckets: observational studies and interventional trials. In an observational study, researchers simply monitor people in their normal settings to identify patterns. For example, a study might track the exercise habits of 10,000 older adults over a decade to see how physical activity correlates with memory retention. These studies are excellent for finding correlations but cannot prove cause and effect.
However, a clinical trial is interventional. This means participants are assigned to a specific treatment or action by the research team to see how it changes their health outcomes. This controlled environment allows scientists to isolate the effects of the treatment from other variables, providing the “proof” required for medical advancement.

These trials aren’t just for testing brand-new “miracle” drugs. They are also used to find new ways to use existing medicines (repurposing), test new diagnostic tools to catch diseases earlier, or explore prevention strategies like vaccines and lifestyle interventions. For instance, many drugs originally approved for blood pressure are now being tested in clinical trials for their potential to treat anxiety or migraines.
Furthermore, clinical trials help establish the “Standard of Care”—the treatment that experts agree is most appropriate for a specific disease. Without the data from these trials, doctors would be forced to rely on intuition or anecdotal evidence, which can be dangerous. At Lifebit, we understand that clinical research is the engine of medical progress. We provide the tech stack that helps researchers manage the massive amounts of genomic and clinical data these studies generate, ensuring that the insights gained are both accurate and actionable. Modern medicine is increasingly moving toward “precision medicine,” where trials are designed to find the right drug for the right patient at the right time, based on their unique genetic makeup.
The Four Phases of Clinical Research: A Deep Dive
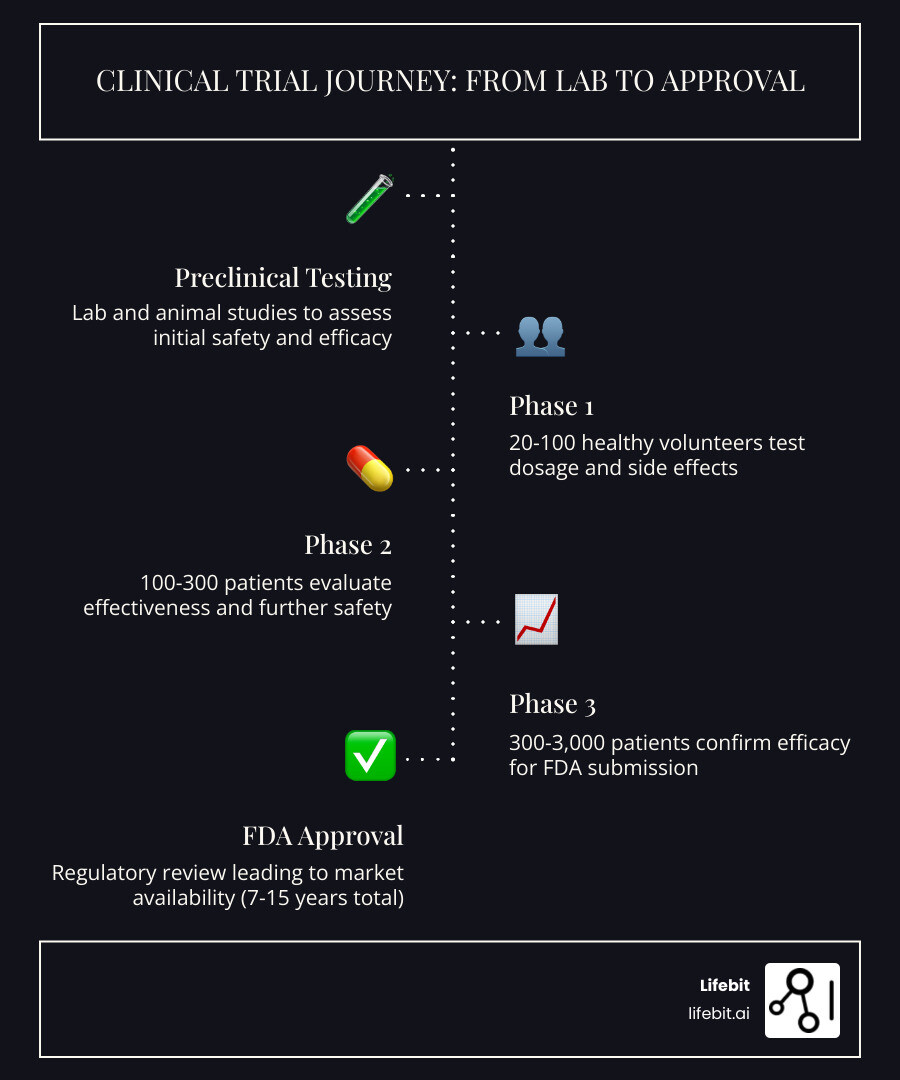
The journey from a lab discovery to your local pharmacy is long, complex, and expensive. On average, it takes 7 to 15 years and can cost billions of dollars to bring a single drug to market. To ensure safety and efficacy, this journey is broken down into four distinct phases, preceded by extensive preclinical testing.
Preclinical Testing: The Foundation
Before any human is ever involved, scientists conduct preclinical tests in laboratories using cell cultures and animal models. The goal is to understand the drug’s basic biology and ensure it isn’t immediately toxic. Only if these results are favorable does the FDA drug review process allow an Investigational New Drug (IND) application to be filed, which is the green light for human testing to begin.
| Phase | Purpose | Participants | Success Rate |
|---|---|---|---|
| Phase 1 | Safety and Dosage | 20–100 Healthy Volunteers | ~70% |
| Phase 2 | Efficacy and Side Effects | 100–300 Patient Volunteers | ~33% |
| Phase 3 | Confirmation and Comparison | 300–3,000 Patient Volunteers | ~25–30% |
| Phase 4 | Post-Marketing Surveillance | Thousands of Real-World Patients | N/A |
What the Phase 1 clinical trial means for safety
Phase 1 is all about the “can we do this?” question. Researchers test a new intervention in a small group of 20 to 100 people. Interestingly, many Phase 1 trials use healthy volunteers rather than people with the specific illness. The goal here is to determine the best dosage, how the body processes the drug (pharmacokinetics), and what the early side effects look like. It’s a vital safety net in medical trials to ensure the treatment isn’t toxic before giving it to a larger group. In some cases, such as oncology, Phase 1 trials may involve patients who have exhausted other options, as the treatments themselves can be quite intense.
Phase 2: Proof of Concept
Once safety is established, Phase 2 focuses on whether the drug actually works for the intended condition. This phase involves several hundred people who have the disease. Researchers look for the “minimum effective dose” and continue to monitor safety. This is often where many drugs fail, as the biological complexity of a disease in humans often differs from what was seen in the lab.
What the Phase 3 clinical trial means for FDA approval
If a drug makes it to Phase 3, it has already shown promise in Phase 2 efficacy tests. Phase 3 is the “big stage.” It involves 300 to 3,000 people and often lasts several years. The goal is to confirm that the drug actually works across a diverse population and to compare it against the current “gold standard” treatment (or a placebo). This phase provides the robust evidence needed for a drug to receive official approval. Ensuring clinical trial success at this stage requires impeccable data integrity, which is where secure, high-performance data platforms become essential. If the results are positive, the company files a New Drug Application (NDA) with the FDA.
Phase 4: Real-World Monitoring
Even after a drug is approved and on the market, the research doesn’t stop. Phase 4 trials, also known as post-marketing surveillance, involve thousands of patients. These studies look for rare side effects that might only appear after millions of people use the drug or to see how the drug performs over many years. This ensures that the long-term safety profile of the medication remains favorable.
Participant Safety: Protocols and Informed Consent
Safety is the absolute priority in any study. The history of clinical research includes dark chapters where participants were not protected, such as the Tuskegee Syphilis Study. These failures led to the creation of the Belmont Report and the Declaration of Helsinki, which established the ethical pillars of modern research: Respect for Persons, Beneficence, and Justice.
Before a trial even starts, researchers must develop a “protocol”—a detailed recipe for the study that explains exactly what will happen, who can join, and how safety will be monitored. This protocol must be approved by an Institutional Review Board (IRB), an independent committee of doctors, statisticians, and community members who ensure the risks to participants are as low as possible and balanced by the potential benefits.
One of the most critical parts of the process is informed consent. This isn’t just a signature on a piece of paper; it’s an ongoing conversation between the participant and the research team. During the consent process, the team must explain in plain language:
- The specific purpose of the study and why it is being conducted.
- The potential risks, side effects, and discomforts that might occur.
- The potential benefits to the participant or to society.
- Exactly what tests, procedures, or hospital visits will be required.
- The right to leave the study at any time without penalty.
It is important to remember that informed consent is not a binding contract. You are a volunteer, and you have the right to withdraw for any reason—whether it’s because of side effects, a change in your health, or simply because you no longer wish to participate. Furthermore, many modern trials utilize Data Monitoring Committees (DMCs). These are independent groups of experts who look at the trial data while the study is ongoing. If they see that a drug is causing unexpected harm, or if it is so effective that it would be unethical to keep it from the control group, they have the power to stop the trial early. We also see a growing trend toward centralized monitoring where AI and digital tools track participant safety in real-time, providing an extra layer of protection that was impossible a decade ago.
Navigating the Risks and Benefits of Enrollment
Deciding to join a trial is a significant personal decision that requires weighing potential medical breakthroughs against known and unknown risks. For many, what a clinical trial means is a chance to access a cutting-edge treatment that isn’t available anywhere else. This is especially true for patients with rare diseases, advanced cancers, or chronic conditions where standard therapies have failed.
Potential Benefits include:
- Access to Innovation: Receiving new treatments before they are widely available to the public.
- Expert Care: Participants are often monitored more closely than they would be in standard care, receiving frequent check-ups and tests from leading specialists.
- Altruism: Contributing to the body of scientific knowledge that will help future generations of patients.
- Cost Savings: In most cases, the study drug and all related medical procedures are provided at no cost to the participant.
Potential Risks include:
- Side Effects: New treatments may have side effects that range from mild (nausea, fatigue) to severe or life-threatening.
- Ineffectiveness: The new treatment might not work for you, or it might be less effective than the current standard of care.
- Time Commitment: Trials often require more frequent doctor visits, blood draws, and travel than regular treatment.
There is also the “randomization” factor. In many Phase 3 trials, participants are randomly assigned to a group by a computer. This is often done in a “double-blind” fashion, meaning neither the patient nor the doctor knows which treatment the patient is receiving. You might receive the new treatment, or you might be in the “control group” receiving the current standard treatment or a placebo (an inactive substance). This design is essential to prevent bias and ensure that the results are scientifically valid.
Researchers use inclusion and exclusion criteria to decide who can participate. These aren’t meant to be “rejections” based on personal factors; they are guidelines to ensure participant safety and scientific accuracy. For example, a study might exclude people with kidney disease if the drug is processed by the kidneys, or include only people within a certain age range to see how a drug affects aging physiology. Finding the right fit is the first step in successful patient recruitment.
The Evolution of Trial Methodology: Observational vs. Interventional
The way we conduct trials is undergoing a digital revolution. Historically, trials were very rigid, centralized, and required everyone to travel to a specific academic hospital, often located in major cities. This created a barrier for people living in rural areas or those with limited mobility. Today, we are seeing the rise of “decentralized clinical trials” (DCTs) that use wearables, mobile apps, and remote monitoring to let people participate from the comfort of their own homes.
This shift is not just about convenience; it’s about data quality and diversity. By using sensors to track heart rate, sleep patterns, or glucose levels in real-time, researchers get a much more accurate picture of how a drug works in the “real world” rather than just during a 15-minute clinic visit.
We are also seeing a massive push for global standards and inclusivity. The WHO resolution on strengthening trials emphasizes that trials should be ethically guided and scientifically robust across the globe, not just in wealthy nations. This is vital because diversity is the “secret sauce” of good science. Genetics, environment, and lifestyle all play a role in how a person responds to a drug. If we only test a drug on one specific demographic, we won’t know if it is safe or effective for everyone else. For example, certain medications for heart disease have been found to work differently in different ethnic groups, a discovery that was only possible through diverse trial populations.
Modern research often involves analyzing “multi-omic” data—looking at everything from your DNA (genomics) to your protein levels (proteomics) and metabolic markers. This generates petabytes of data that must be analyzed securely. At Lifebit, we specialize in decentralized clinical trials and federated data analysis. This technology allows researchers to study diverse populations across different countries without ever moving sensitive patient data out of its secure home, maintaining the highest levels of privacy while accelerating the pace of discovery. We are moving toward a future where “In Silico” trials—using computer simulations and virtual patients—might supplement human testing to further increase safety and speed.
Frequently Asked Questions about Clinical Trials
How do I find a clinical trial near me?
The best place to start is ClinicalTrials.gov, a massive database maintained by the National Library of Medicine that lists studies happening in over 200 countries. You can search by your specific condition, location, or even search for “healthy volunteer” studies if you want to contribute to science without having a specific illness. You should also talk to your primary doctor or specialist, as they often have access to local registries or know of upcoming studies in their field. If you’re looking for clinical studies near me, remember to check the eligibility criteria carefully before reaching out to the research coordinator.
Can I withdraw from a trial after it starts?
Yes, absolutely. One of the fundamental ethics of withdrawal is that participation is entirely voluntary. You can stop participating at any time, for any reason, and it will not affect your standard medical care, your insurance coverage, or your relationship with your doctor. The research team may ask to perform a final safety check-up, but you are under no obligation to continue the intervention.
Why is diversity important in research?
Diversity is not just a buzzword; it’s a scientific necessity. Different groups of people (based on age, race, ethnicity, and sex) can react differently to the same medication due to biological and environmental factors. For example, the elderly consume one-third of all drugs but are often excluded from trials due to co-existing conditions, leading to a lack of data on how these drugs affect older bodies. The FDA diversity guidance now encourages researchers to include a wider range of participants to ensure that the results of a study apply to the whole population, not just a small slice of it.
What is “Compassionate Use” or “Expanded Access”?
Sometimes, if a patient has a life-threatening condition and no other treatments are available, they may be able to access an investigational drug outside of a clinical trial. This is known as “Expanded Access” or “Compassionate Use.” It requires approval from both the drug manufacturer and the FDA, and it is typically reserved for cases where the patient cannot join an existing trial.
Does it cost money to be in a clinical trial?
In the vast majority of cases, there is no cost to the participant. The pharmaceutical company or government agency sponsoring the trial pays for the study drug, the medical tests, and the doctor visits. Some trials even offer a small stipend for time and travel expenses. It is always important to ask the research coordinator about any potential hidden costs during the informed consent process.
Conclusion: Powering the Future of Research
Understanding what a clinical trial means is about recognizing the human element in science. It’s about the courage of volunteers who step into the unknown and the dedication of researchers working together to solve the world’s toughest health challenges. From the first Phase 1 safety test to the final Phase 4 real-world monitoring, every step is designed to ensure that when a doctor writes a prescription, they are doing so based on hard evidence and proven safety.
As we move into an era of personalized medicine and AI-driven discovery, the nature of clinical trials will continue to change. We will see more trials tailored to a person’s specific genetic code and more studies that use real-world data to supplement traditional clinical evidence. This evolution promises to make treatments more effective and reduce the time it takes to bring new hope to patients.
At Lifebit, we are proud to play a role in this ecosystem. Our federated AI platform provides secure, real-time access to global biomedical data, enabling researchers to collaborate across five continents without compromising patient privacy. By providing tools like our Trusted Research Environment (TRE) and Real-time Evidence & Analytics Layer (R.E.A.L.), we help turn complex clinical data into the medical breakthroughs of tomorrow. We believe that by breaking down data silos, we can make the clinical trial process faster, fairer, and more effective for everyone.
To learn more about how we are accelerating the future of medicine and supporting the next generation of clinical research, explore the Lifebit platform.

