How Data Science is Saving the Future of Clinical Trials

Why the Future of Clinical Trials Must Change Now
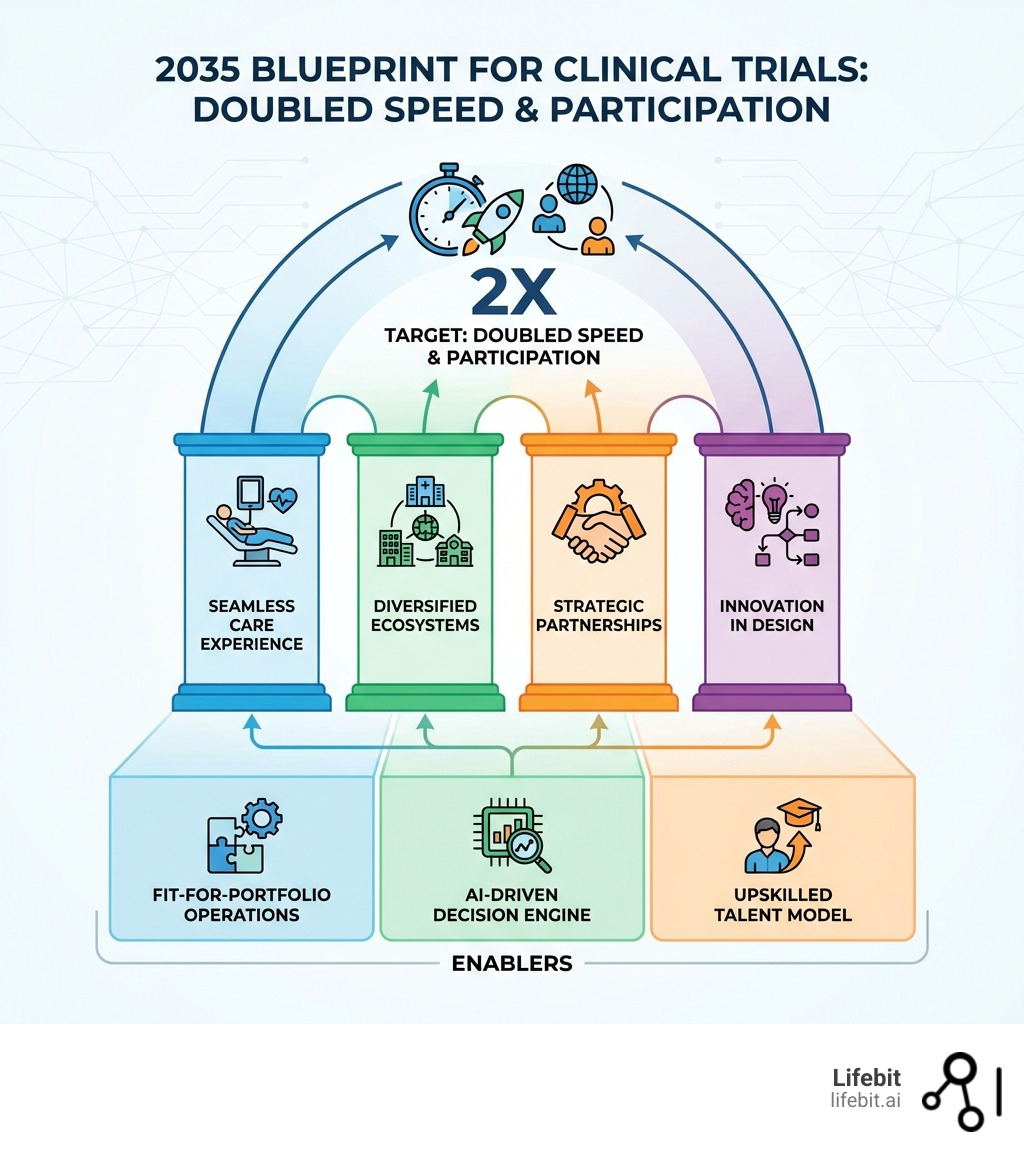
The future of clinical trials is arriving faster than most organizations are prepared for. By 2035, the industry aims to double trial speed and patient participation while simultaneously improving outcomes and reducing costs. This transformation is being driven by five critical shifts:
- Patient-centric care integration — Trials embedded into everyday healthcare settings, eliminating travel burdens
- AI-powered trial design — Adaptive protocols, digital twins, and automated decision-making replacing static methodologies
- Diversified trial ecosystems — Community sites and hub-and-spoke models expanding access beyond academic centers
- Strategic site-sponsor partnerships — Long-term, data-driven relationships replacing transactional engagements
- Regulatory modernization — ICH E6(R3) and federated data governance enabling real-time compliance and global collaboration
The stakes are enormous. Phase III trials have increased 2.5 times since 2000, yet most patients still cannot access trials where they receive care. Only 10% of schizophrenia patients in the United States can participate at their current clinic. 60-80% of patients with heart failure, ulcerative colitis, sickle cell anemia, and cystic fibrosis face the same barrier. Meanwhile, regulatory bodies demand tighter data integrity, AI adoption accelerates without clear guardrails, and fragmented processes waste billions annually.
The clinical research industry is at a critical juncture. Organizations that fail to integrate federated AI platforms, real-time analytics, and patient-driven models will struggle to compete in a landscape where speed, equity, and compliance determine success.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over a decade building federated AI platforms that power the future of clinical trials across secure, compliant environments for global pharma and public sector institutions. Below, I’ve gathered insights from leading experts on how data science is reshaping every aspect of clinical research by 2035.
Handy future of clinical trials terms:
Why the Future of Clinical Trials Depends on Seamless Patient Care

For decades, clinical trials have operated as an “extra” activity, often physically and logistically separated from standard medical care. This separation is the primary cause of the participation crisis we see today. When we look at the data, the geographical barriers are staggering. About one-third of schizophrenia patients must drive more than 60 minutes just to access a trial site. For many, this isn’t just an inconvenience—it’s a disqualifier. Research shows that for every 30 minutes of additional travel time, patient retention rates drop by nearly 20%, creating a massive attrition problem that delays drug approvals by months or even years.
To fix this, the future of clinical trials must move into the patient’s existing care circle. Imagine a world where a patient with heart failure or cystic fibrosis doesn’t have to choose between their trusted local doctor and a potentially life-saving experimental therapy. By integrating trials into standard care, we can reduce the “white spaces” in trial access—those geographic gaps like Chico, California, or Lafayette, Louisiana, where patients currently have almost no local options. This involves empowering community physicians with the tools to act as sub-investigators, allowing them to oversee trial protocols without requiring the patient to travel to a distant academic medical center (AMC).
This shift requires a new understanding of AI in healthcare regulatory landscapes. Regulators are beginning to recognize that data collected in a real-world setting, if managed with high integrity, can be just as robust as data from a sterile academic center. The use of Electronic Health Record (EHR) integration allows for the seamless capture of trial data during routine visits, reducing the administrative burden on local clinics and ensuring that the “real-world” experience of the patient is accurately reflected in the study results.
Decentralized Models and the Future of Clinical Trials
The “hub-and-spoke” model is the architectural backbone of this new era. In this setup, a central academic “hub” provides the scientific oversight, specialized equipment, and principal investigator expertise, while community “spokes”—local clinics, pharmacies, and even at-home care providers—handle the day-to-day patient interaction. This model effectively democratizes access to innovation. For example, a patient in a rural area could receive their initial screening at a local CVS or Walgreens, have their medication delivered to their home, and use wearable devices to monitor vital signs, all while being overseen by a world-class specialist at a hub hundreds of miles away.
This diversification is essential for health equity. By leveraging decentralized clinical trial models, we can reach indigenous and minority populations who have historically been excluded from research due to systemic barriers and lack of proximity to major research institutions. We aren’t just talking about convenience; we’re talking about making sure the medicines of tomorrow work for everyone, not just those who live near a major university hospital. Representation in clinical trials is a scientific necessity; without diverse data, we cannot be certain of a drug’s efficacy across different genetic backgrounds and lifestyles.
Fit-for-Portfolio Operations
Not all trials are created equal. A Phase III trial for a chronic condition like diabetes requires a vastly different operational setup than a Phase I trial for an ultra-rare genetic disease. The future of clinical trials demands “fit-for-portfolio” models that move away from the rigid, one-size-fits-all approach of the past:
- Rare Diseases: Focus on “n-of-1” designs and high-touch patient support. These trials often require global recruitment because the patient population is so small. Here, the focus is on concierge-level logistics to bring the trial to the patient, wherever they are in the world.
- Chronic Conditions: Focus on scale, leveraging remote monitoring and at-home care to maintain high enrollment. For conditions like hypertension or asthma, the goal is to make the trial as invisible as possible in the patient’s daily life, using passive data collection through smartphones and wearables.
- Oncology: Requires a hybrid approach where complex infusions happen at a specialized center, but follow-up monitoring and blood work are handled locally. This reduces the “toxicity of travel” for patients already dealing with the physical toll of cancer treatment.
By tailoring the operations to the specific disease type, sponsors can stop wasting resources on “one-size-fits-all” infrastructure that serves no one well, ultimately lowering the cost of drug development and speeding up the time to market.
Accelerating Development with AI-Optimized Designs and Digital Twins
The traditional trial protocol is a static document, often hundreds of pages long, that remains unchanged even when early data suggests a better path. This rigidity is a relic of a pre-digital age. That is changing. Adaptive protocols now allow us to expand enrollment in treatment arms that show early promise and quickly drop those that don’t. This “fail fast” or “succeed faster” mentality is crucial for managing the rising costs of R&D. By using Bayesian statistical models, researchers can adjust sample sizes or dosage levels in real-time based on interim results, ensuring that the trial remains both scientifically rigorous and ethically sound.
But the real game-changer is the “Digital Twin.” By using generative molecular design and historical patient data, we can create virtual models of patients. These digital twins can act as a synthetic control arm, potentially reducing the number of real patients who need to be assigned a placebo. This is particularly transformative in pediatric or terminal illness trials where assigning a patient to a placebo arm is ethically difficult. A synthetic control arm uses high-fidelity historical data from previous trials and real-world evidence to simulate how a control group would respond, allowing more participants to receive the active treatment while still maintaining a valid comparison group.
How Agentic AI Transforms the Future of Clinical Trials
We are moving past simple AI that just “suggests” things. The future of clinical trials belongs to agentic AI—intelligent systems that can autonomously manage complex workflows and make data-driven decisions within predefined guardrails. These agents act as a digital nervous system for the trial, connecting disparate data points into a cohesive strategy.
- Site Selection: AI analyzes years of performance data, local disease prevalence, and even local infrastructure (like proximity to public transport) to find sites that actually recruit on time. This moves site selection from a “who you know” relationship model to a “what the data shows” performance model.
- Patient Matching: LLMs scan clinical trial patient data and unstructured EHR notes to find the perfect candidates in seconds, not months. This eliminates the manual chart review process that currently consumes thousands of hours of site staff time.
- Workflow Automation: Managing the 700+ steps required to initiate a Phase III oncology trial can now be handled by AI agents that coordinate between sites, labs, and sponsors. These agents can automatically flag delays, trigger resupply of investigational products, and ensure that all regulatory documents are up to date.
- Predictive Analytics for Retention: AI can identify patterns in patient behavior that suggest a high risk of dropping out—such as missed app check-ins or delayed responses—allowing trial coordinators to intervene with personalized support before the patient leaves the study.
Generative AI Safeguards
With great power comes great responsibility (and regulatory scrutiny). The integration of generative AI brings risks like “hallucinations” or the reinforcement of historical biases in training data. If an AI is trained on data from a population that lacks diversity, its “optimized” trial design may inadvertently exclude certain groups. To counter this, the FDA guidelines on AI in drug development emphasize transparency and “explainability.”
We must implement tiered oversight. A tool that helps draft a protocol needs less scrutiny than an AI agent making decisions about patient safety or dose escalation. At Lifebit, we advocate for secure data platforms that allow AI to learn from data without the data ever leaving its secure environment. This “federated” approach ensures that sensitive patient information remains private while still allowing the AI to gain the insights necessary to optimize the trial. By keeping the data behind the hospital’s or the sponsor’s firewall, we mitigate the risks of data breaches and ensure compliance with global privacy laws like GDPR and HIPAA.
Navigating Regulatory Shifts and ICH E6(R3) Compliance
The regulatory landscape is undergoing its most significant update in a generation. The adoption of ICH E6(R3) is shifting the focus from “checking boxes” to “Quality by Design” (QbD). This means compliance isn’t something you do at the end of a trial; it’s something you bake into the data strategy from day one. QbD requires sponsors to identify the “critical to quality” factors of their trial—the specific data points and processes that are essential to patient safety and the reliability of the results—and build robust systems to protect them.
A major focus of this new era is biospecimen data integrity. Regulators now want to see the “chain of custody” for every sample, from the moment it leaves a patient’s arm to its final analysis in a lab. In the past, this was often a fragmented process involving multiple vendors and manual logs. The future requires integrated, vendor-agnostic technology that can track metadata across multiple systems without adding burden to the research sites. This ensures that when a regulator asks for the provenance of a specific biomarker result, the answer is available in seconds, not weeks.
Compliance is also being shaped by high-level policy, such as the Executive Order on Safe AI, which demands that AI tools used in research are trustworthy, secure, and unbiased. This means that the “black box” approach to AI is no longer acceptable. Sponsors must be able to demonstrate how their AI arrived at a specific conclusion, particularly when it affects trial endpoints or patient eligibility.
Global Harmonization and Data Strategy
The current trial landscape is fragmented. Every country, and sometimes every hospital, has its own way of managing data. This “data silo” problem throttles innovation. The future of clinical trials depends on global harmonization—standardizing how we collect, store, and share information. Without this, the dream of a truly global, real-time trial ecosystem remains out of reach.
By using federated data integration, we can allow researchers to query global datasets without the logistical nightmare of moving massive files across borders. This is the “always on, always busy” vision proposed by the WHO: a global infrastructure that stays ready for the next health crisis while accelerating daily research. Federated learning allows models to be trained on data at its source. For example, a researcher in London could run an analysis on patient data in Tokyo and New York simultaneously. The data stays local, satisfying strict national data sovereignty laws, but the insights are shared globally. This approach is the only way to achieve the scale required for the next generation of precision medicine, where we may be looking for a specific genetic mutation that only exists in a handful of people worldwide.
Scaling Success: Regional Strategies and Talent Evolution
Regional hubs are already showing us the way. British Columbia, for example, conducts over 1,300 trials a year and has the fastest-growing life sciences sector in Canada. Their secret? A shared provincial vision that aligns clinical research with standard care and economic growth. By creating a unified ethics review process and a single point of entry for sponsors, they have drastically reduced the time it takes to get a trial up and running. This regional approach serves as a blueprint for other jurisdictions: when the government, healthcare providers, and industry work together, the entire ecosystem thrives.
However, technology is only half the battle. We also need to rethink who is running these trials. The role of the Clinical Research Associate (CRA) is evolving from a “data monitor” who travels from site to site checking source documents to a “strategic relationship manager” who uses real-time analytics to oversee trial health. This shift requires a massive upskilling of the workforce. We need professionals who are as comfortable with data science and AI as they are with clinical protocols.
| Traditional Requirement | 2035 Talent Requirement |
|---|---|
| Manual data verification and source document review | Proficiency in AI-driven analytics and remote monitoring tools |
| Transactional site visits focused on compliance checklists | Strategic site partnership management and performance coaching |
| Rigid protocol adherence with little room for adjustment | Adaptive design management and real-time problem solving |
| Generalist focus across all therapeutic areas | Specialized roles (e.g., Patient Liaison, Digital Recruitment, AI Ethics Officer) |
| Paper-based or siloed digital systems | Expertise in federated data environments and integrated EHR systems |
The Economic Value of Speed
The push for doubling trial speed isn’t just about patient health; it’s an economic imperative. It is estimated that every day a drug’s market entry is delayed, the sponsoring company loses between $600,000 and $8 million in potential revenue. More importantly, for patients with progressive diseases, a one-year delay in a trial can mean the difference between a manageable condition and a terminal one. By 2035, the goal is to reduce the average drug development timeline from 10-12 years down to 5-7 years. This will be achieved not by cutting corners, but by eliminating the “dead time” in the process—the months spent waiting for site contracts, the weeks spent on manual data entry, and the years spent on inefficient patient recruitment. The future of clinical trials is one where every minute is treated as a precious resource.
Frequently Asked Questions about Clinical Research Shifts
What are the primary forces reshaping clinical trials by 2035?
The main drivers are therapeutic innovation (like gene and cell therapy), the surge in Phase III trial volume (which has more than doubled since 2000), geographic shifts toward more diverse populations, and the “lightspeed” benchmarks set during the COVID-19 pandemic. Additionally, the rising cost of traditional trials is forcing a shift toward more efficient, AI-driven methodologies.
How will AI-optimized endpoints reduce trial costs?
By using AI to identify more precise biomarkers and surrogate endpoints, trials can achieve statistical significance with fewer patients and in less time. For example, instead of waiting five years to see if a drug prevents heart attacks, AI might identify a specific protein change that occurs in six months and reliably predicts the long-term outcome. Reducing the need for massive, multi-year trials for every indication can save hundreds of millions in development costs.
What role does health equity play in future trial designs?
Health equity is no longer an “optional” goal or a PR exercise. Regulators increasingly require trial populations to reflect the actual diversity of the patients who will use the drug. Increasing diversity in clinical trials is now a core component of successful regulatory submissions. This requires moving trials into community settings and using decentralized tools to reach underserved populations.
What is a “Digital Twin” in the context of clinical research?
A Digital Twin is a virtual representation of a patient, created using their historical health data, genetic information, and real-world evidence. In a trial, these twins can be used to simulate how a patient would respond to a treatment or a placebo. This allows for “in silico” testing before or alongside human trials, improving safety and potentially reducing the number of human subjects needed for a study.
How does federated AI protect patient privacy?
Federated AI allows an algorithm to be trained across multiple decentralized servers (like different hospitals) without the need to exchange the actual data. The AI “travels” to the data, learns from it, and then shares only the learned insights (the “weights”) with a central model. This ensures that the raw patient data never leaves its original, secure location, maintaining 100% compliance with privacy regulations like GDPR.
Conclusion: Taking Action for the 2035 Vision
The future of clinical trials isn’t a distant dream; it’s a blueprint being built today. To reach the goal of doubling speed and participation by 2035, stakeholders must act now:
- Sponsors: Shift from transactional site relationships to long-term strategic partnerships.
- Sites: Adopt “site-friendly” integrated technologies that reduce administrative burden.
- Regulators: Continue the push toward global harmonization and real-time monitoring.
At Lifebit, we are proud to provide the federated AI platform that makes this vision possible. By enabling secure, real-time access to global biomedical data, we help biopharma and governments collaborate without compromise. The road to 2035 is paved with data—let’s make sure we use it to save lives, faster.
Learn more about how Lifebit is powering the future of research.

