Beyond the Buzz: What Every Researcher Needs to Know About Clinical Data Portals

Stop Drowning in Data Silos: How Portals Open up Faster Medical Research
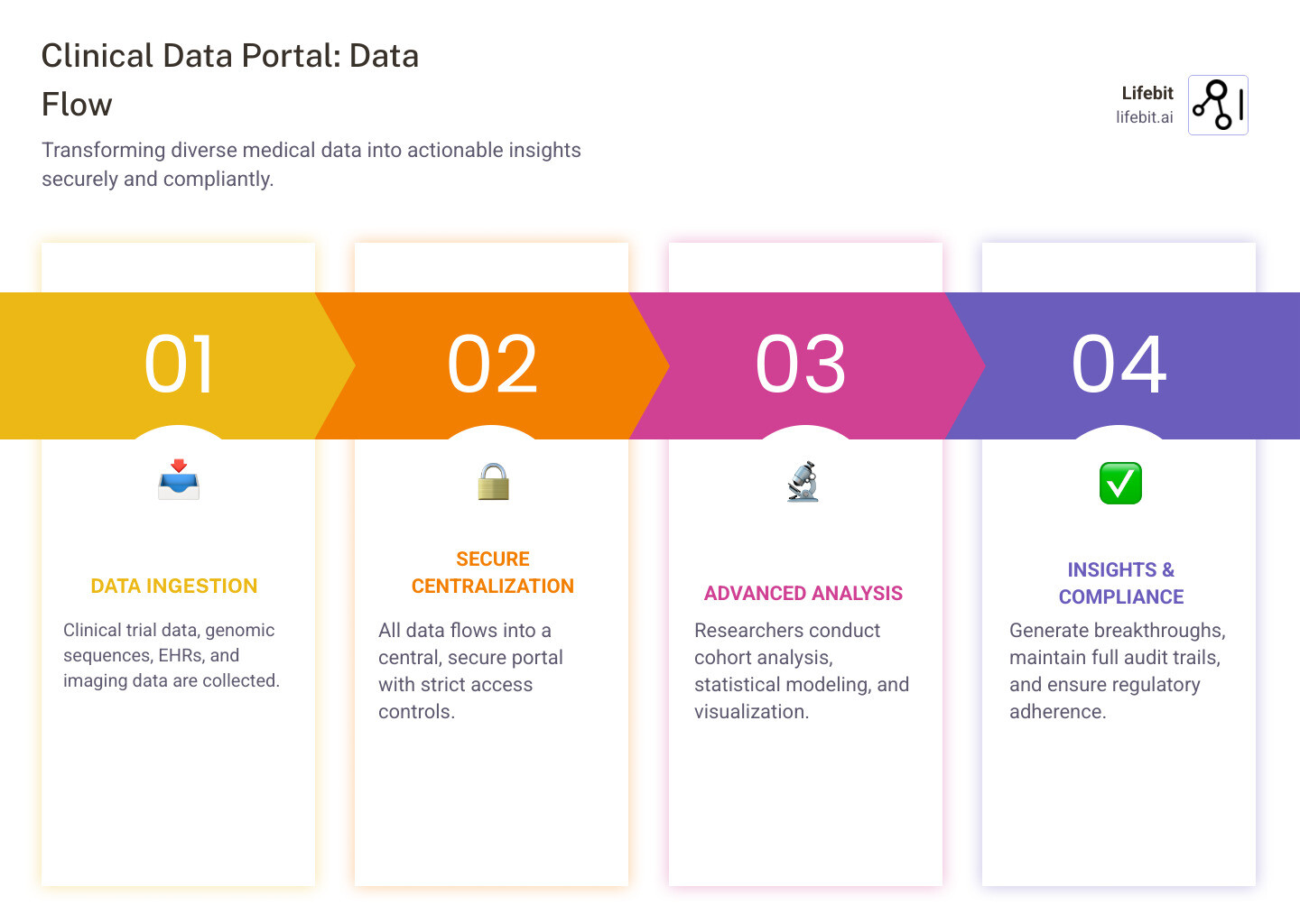
A clinical data portal is a secure, centralized platform that enables researchers, healthcare providers, and patients to access, search, and analyze clinical trial data, genomic information, and other biomedical datasets. By aggregating diverse data sources into a single access point, these portals accelerate research, improve patient outcomes, and ensure regulatory compliance.
Key features of clinical data portals include:
- Centralized data access for trials, genomics, imaging, and EHRs
- Advanced search and filtering to find relevant cohorts or datasets
- Secure collaboration with controlled data sharing and full audit trails
- Built-in analytical tools for cohort building, visualization, and analysis
- Regulatory compliance with standards like HIPAA, GDPR, and GxP
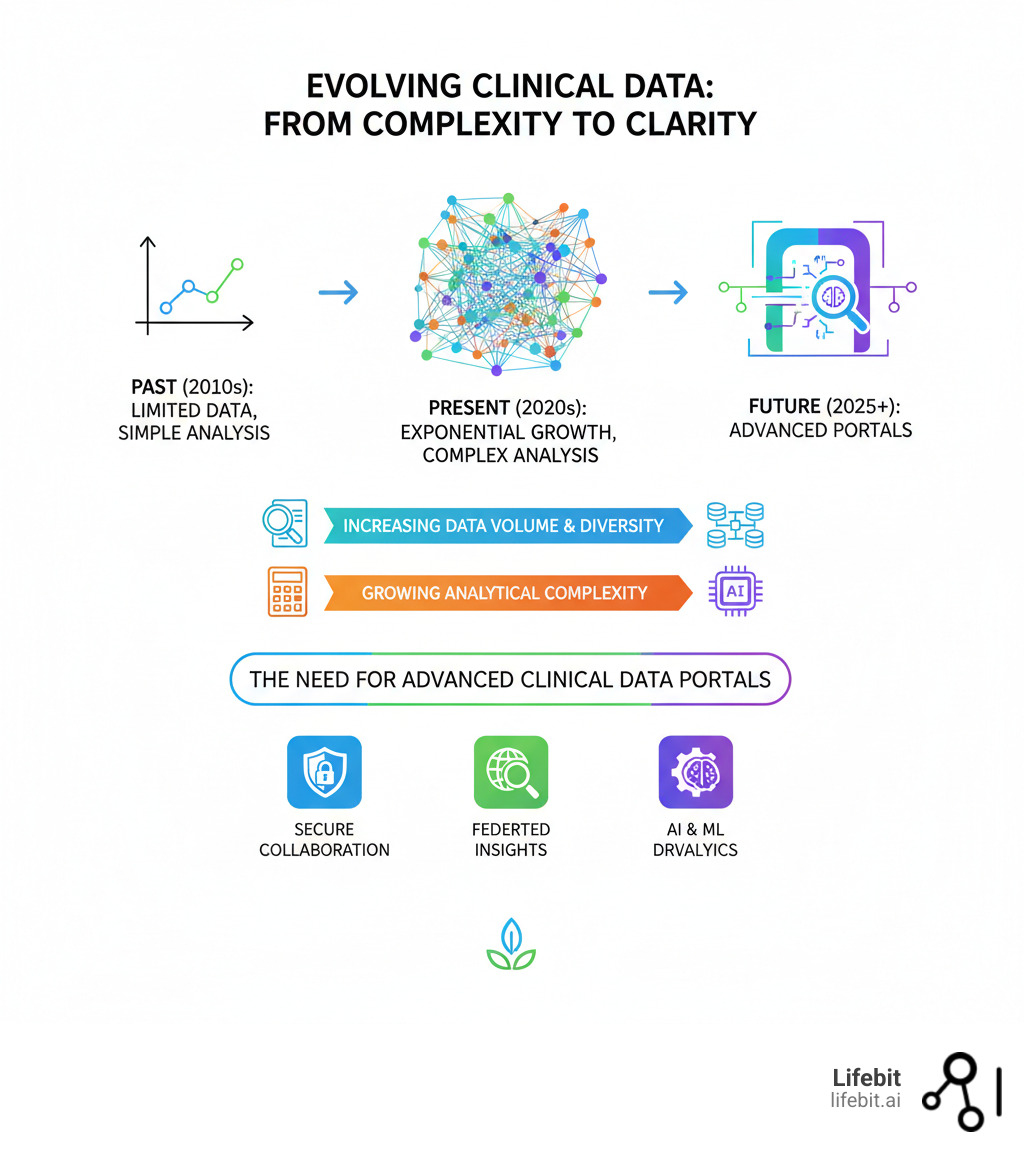
The global research landscape is shifting. Major platforms like the Genomic Data Commons host data from over 45,000 cases, and the European Medicines Agency now provides broad access to clinical data from pharmaceutical companies. This transparency is reshaping drug development.
Yet, most organizations still struggle with fragmented data silos, slow onboarding, and analytics that require moving sensitive data. Traditional systems weren’t built for the scale of modern genomic and multi-modal datasets.
As CEO and Co-founder of Lifebit, I’ve seen how secure, federated analysis can open up insights from siloed biomedical data. The shift toward clinical data portals is more than a tech trend—it’s a fundamental change in how we generate evidence and improve patient outcomes.
Find Life-Saving Data in Days, Not Months
Picture a researcher hunting for a gene mutation linked to a rare cancer. Without a portal, they face months of paperwork and data wrangling. A clinical data portal solves this. It’s a universal translator for the medical data universe—a secure platform that brings genomic sequences, patient records, and more into one searchable, analysis-ready place.
The goal is to make health data findable, accessible, and usable. When data becomes a living resource, breakthrough treatments reach patients faster. That’s not just convenient—it’s life-changing. For organizations building this infrastructure, our Complete Guide to Data Integration Platforms explores the technical foundations.
The Core Function of a Clinical Data Portal
A clinical data portal streamlines research by:
- Aggregating data: It pulls information from hospitals, labs, and trial databases into a coherent structure.
- Centralizing access: Researchers log into one secure environment, focusing on science, not system administration.
- Enabling advanced search: Users can build precise cohorts in minutes by filtering on diagnosis, biomarkers, or treatment history.
- Providing analysis tools: Portals act as research workbenches, allowing visualization and statistical analysis without moving sensitive data.
- Fostering collaboration: Teams can work on the same datasets with granular access controls, which is essential for multi-institutional studies.
Our overview of Clinical Research Data Software can help you evaluate key features.
Unpacking the Data: From Genomics to Patient Records
A clinical data portal manages the full spectrum of biomedical data:
- Genomic and Proteomic Data: The backbone of precision medicine, this includes vast datasets like whole-genome sequences (WGS), which map an individual’s entire DNA, and whole-exome sequences (WES), which focus on protein-coding regions. It also includes transcriptomic data (e.g., RNA-Seq) that reveals gene expression patterns. These datasets are enormous—a single human genome can be over 100 gigabytes—and require specialized bioinformatics tools for analysis. Portals must manage these massive files (like BAM, VCF, and FASTQ) and link them to clinical outcomes. Proteomic data, which measures the abundance and modification of proteins, adds another layer of biological complexity, helping researchers understand how genetic information is translated into cellular function. The Genomic Data Commons, for example, hosts data on over 3 million mutations, providing the scale needed for meaningful discovery.
- Electronic Health Records (EHRs): The day-to-day reality of patient care, EHRs contain a wealth of information, including diagnoses, medications, lab results, and physician’s notes. A major challenge is that this data exists in both structured (e.g., ICD-10 codes, discrete lab values) and unstructured formats (e.g., free-text clinical notes). Advanced portals often incorporate Natural Language Processing (NLP) tools to extract meaningful information from unstructured text, turning narrative descriptions into analyzable data points.
- Diagnostic Imaging: Medical images like X-rays, MRIs, and CT scans add crucial visual context. The volume of this data is staggering, and portals must handle the standard DICOM (Digital Imaging and Communications in Medicine) format. Increasingly, these images are not just viewed by radiologists but are analyzed by AI algorithms to detect patterns invisible to the human eye, a field known as computational pathology. A portal can serve as the repository for both the images and the AI-derived analytical results.
- Clinical Trial and Real-World Data (RWD): This category includes structured data from formal studies and a growing torrent of information from everyday clinical practice, known as Real-World Data (RWD). RWD sources include insurance claims databases, patient registries, and even data from mobile health apps and wearables. This data is gold for understanding long-term treatment efficacy, safety, and cost-effectiveness outside the controlled environment of a clinical trial. Explore our guide on Clinical Trial Patient Data for more.
- Phenotypic Information: These are the observable traits of an individual, such as height, weight, blood pressure, and disease symptoms or progression. Phenotypic data is the critical link that connects genetic and molecular data to real-world health outcomes. A portal’s power lies in its ability to let a researcher query across these data types, for example, to find all patients with a specific gene variant, a history of high blood pressure, and a particular outcome in a clinical trial.

The Triple Win: How Connected Data Accelerates Research, Improves Care, and Empowers Patients
Clinical data portals don’t just solve one problem—they create a triple win for researchers, clinicians, and patients. By breaking down data silos, they accelerate the entire healthcare ecosystem.

For Researchers: Accelerating the Pace of Innovation
Instead of spending months negotiating data access and dealing with administrative hurdles, researchers can dive straight into science. For example, a team studying a rare form of pediatric cancer can use a portal to instantly identify a cohort of patients with a specific genetic fusion (e.g., NTRK), access their anonymized treatment histories, and analyze their tumor’s RNA sequencing data to understand why some responded to a targeted therapy while others did not. This entire discovery process, which could have taken years, is condensed into weeks or months.
This access also enables powerful secondary analysis—extracting new insights from existing data, which dramatically reduces costs and maximizes the value of each participant’s contribution. Cohort building becomes exponentially faster, as researchers can build and refine complex queries across thousands of cases in minutes. Most importantly, centralized portals improve reproducibility, a cornerstone of good science. By providing access to versioned datasets and containerized analytical environments (using technologies like Docker), they ensure that a study can be precisely replicated by other scientists, validating the findings. Our Complete Guide to Clinical Trial Data explores how this standardization drives better outcomes.
For Healthcare Providers: Enhancing Clinical Decision-Making
In an emergency, a clinician needs a patient’s full history instantly. Clinical data portals provide this comprehensive, longitudinal view, connecting records from multiple hospitals, clinics, and labs to show a complete picture of medications, allergies, past diagnoses, and recent tests. This helps avoid dangerous drug interactions and redundant testing, saving time, money, and improving patient safety. Beyond immediate care, these portals can power sophisticated Clinical Decision Support Systems (CDSS). For instance, a CDSS integrated with a portal could analyze a cancer patient’s genomic profile from their EHR and automatically flag them as an eligible candidate for a new clinical trial, presenting this option directly to their oncologist. This transforms the portal from a passive data repository into an active tool for delivering personalized, efficient, and informed care.
For Patients: Empowering Participation and Transparency
Portals are putting information and power back into patients’ hands. The ICTRP Search Portal, for example, provides a single point of access to information about ongoing and completed clinical trials globally, connecting patients with cutting-edge therapies.
This transparency allows patients and families to make informed decisions about their care. When patients share their de-identified data, they contribute to findies that will benefit countless others. The FDA even provides information for patients on Expanded Access to unapproved medical products. This collaborative cycle is the future of medicine.
4 Types of Clinical Data Portals Driving Global Research
The world of clinical data portals is diverse, with different types serving specific purposes. Some track every trial globally, while others dive deep into genomic data. Together, they form the infrastructure reshaping medical knowledge. Let’s tour some of the most influential examples.

Global Trial Registries
These portals are the first stop for finding information on clinical trials. The ICTRP Search Portal from the WHO acts as a master index, pulling data from registries worldwide to prevent duplicate studies and identify research gaps. ClinicalTrials.gov is another key resource, hosting detailed information on trial design, status, and results from over 220 countries.
Large-Scale Genomic & Health Repositories
These portals house the actual biological and clinical data. The Genomic Data Commons (GDC) harmonizes cancer data from landmark studies, allowing researchers to build custom cohorts based on specific genetic mutations. UK Biobank tracks half a million UK participants over time, collecting genetic, lifestyle, and imaging data to understand how genes and environment influence health.
Regulatory & Government-Led Portals
Government portals ensure the data behind approved drugs is accessible. The European Medicines Agency (EMA) was the first to provide public access to the detailed clinical study reports submitted by pharmaceutical companies. In Canada, Health Canada’s Clinical Trials Database lists authorized trials, helping Canadians find studies to participate in.
| Portal Type | Example | Purpose | Data Hosted | Primary Users |
|---|---|---|---|---|
| Global Trial Registry | ClinicalTrials.gov | Comprehensive trial tracking | Trial design, status, results, contact information | Patients, healthcare providers, researchers |
| Global Trial Registry | ICTRP Search Portal | Unified global trial access | Aggregated trial registration data | Researchers, public, policymakers |
| Genomic Repository | Genomic Data Commons (GDC) | Deep cancer research | Harmonized genomic and clinical data | Computational biologists, cancer researchers |
| Health Repository | UK Biobank | Long-term health studies | Genetic, imaging, clinical, lifestyle data | Geneticists, epidemiologists, health researchers |
| Regulatory Portal | EMA Clinical Data Publication | Drug approval transparency | Clinical study reports, regulatory submissions | Public, researchers, regulators |
| Regulatory Portal | Health Canada’s Clinical Trials Database | Trial authorization tracking | Authorized trial information | Canadian public, researchers |
These different portal types complement each other, allowing researchers to move from trial findy to deep data analysis and regulatory review, piecing together a complete picture.
The 3 Biggest Problems to Secure Data Sharing (And How to Clear Them)
Building a clinical data portal that people trust is complex. Health data carries enormous responsibility, and portals must balance innovation with protection, speed with safety, and openness with privacy. Our framework for Clinical Data Governance addresses these critical concerns.
Ensuring Data Privacy and Security
Protecting sensitive information like genetic data or mental health records requires a multi-layered defense. The first step is often de-identification and anonymization, stripping away direct personal identifiers like name and address. However, re-identification can still be possible, so advanced techniques like differential privacy are sometimes used, which add mathematical noise to query results to protect individual privacy while preserving statistical accuracy. Governance is managed through Data Access Committees (DACs), independent bodies that review researcher applications, ensuring that data is used ethically and for scientifically valid purposes. Access is then granted based on the principle of least privilege, where users only see the specific data they are authorized for.
All this happens within secure data environments (SDEs), which are highly controlled computing platforms. These environments use encryption for data at rest and in transit, robust user authentication, and continuous intrusion detection. Critically, analysis happens inside the SDE, so sensitive information never has to be moved or downloaded. Researchers bring their tools and code into the environment through secure ‘airlocks,’ and only non-sensitive, aggregated results can be exported, ensuring the raw data remains protected. Our guide on What is a Secure Data Environment (SDE)? explores these safeguards. Finally, all portals must operate within strict compliance frameworks like HIPAA in the US and GDPR in Europe.
The Challenge of Data Standardization and Interoperability
A “fever” recorded at one hospital may not mean the same thing as “pyrexia” at another. This lack of a common language makes combining data a massive challenge. For example, one EHR might code “Type 2 Diabetes” as “T2DM,” another might use the ICD-10 code “E11,” and a third might only mention it in a physician’s free-text notes. Without standardization, a query for diabetic patients would miss many of them. Data harmonization is the solution—a painstaking process of mapping and converting data to common formats, units, and vocabularies.
Common Data Models (CDMs) like OMOP and CDISC provide standardized database schemas, while standardized terminologies like LOINC (for lab tests) and SNOMED CT (for clinical findings) provide the shared vocabulary. A key emerging standard is FHIR (Fast Healthcare Interoperability Resources), which defines a modern, API-based way for different systems to exchange healthcare information. Achieving this interoperability is a major cultural and technical challenge, but the payoff is the ability to unlock the full power of aggregated data. Our Complete Guide to Clinical Data Interoperability dives deeper into these solutions.
The Role of Regulatory Bodies
Regulatory bodies like the FDA and EMA act as traffic engineers, ensuring data sharing runs safely. Data submission mandates require trials to be registered and results published, preventing selective reporting. The EMA’s transparency policy was revolutionary, giving independent researchers access to the same data regulators use to approve drugs.
Regulators also perform rigorous quality control and enforce compliance with GxP (Good Practice) quality guidelines and rules like 21 CFR Part 11 for electronic records. These regulations mandate features like secure, computer-generated, time-stamped audit trails to track all data changes, the use of legally binding electronic signatures, and strict access controls. These requirements ensure the data you access is complete, trustworthy, and has not been tampered with. For European researchers, the Clinical Trials in the European Union – EMA portal is a prime example of how regulatory oversight and public access can coexist.
The Future of Clinical Data: 3 Trends You Can’t Ignore
The evolution of clinical data portals is accelerating. The future isn’t just about bigger databases; it’s about smarter, federated systems that generate evidence in real time while keeping data secure where it lives.
1. AI and Machine Learning Integration
The massive datasets in clinical data portals are perfect for AI and Machine Learning. Predictive modeling can forecast disease progression or identify at-risk patients. Automated cohort building allows ML algorithms to find specific patient populations in minutes, not weeks. And advanced analytics can connect dots across genomic, imaging, and clinical data to reveal new therapeutic targets and biomarkers.
2. The Rise of Federated and Secure Research Environments
The old model of moving sensitive patient data to a central location for analysis is becoming obsolete due to privacy risks, data sovereignty laws, and the sheer size of the datasets. Federated learning flips the script: instead of bringing data to the analysis, the analysis is brought to the data. This is the core principle of Trusted Research Environments.
In a federated network, an analysis query or machine learning model is sent to each participating institution’s secure, local environment. The analysis runs locally on the data, which never leaves the institution’s firewall. Only the aggregated, non-identifiable results or model updates are returned to a central coordinator. This process enables powerful multi-party collaboration across countries, as researchers can generate insights from combined datasets that could never be physically merged. This approach also directly overcomes data sovereignty challenges, respecting laws that prevent health data from leaving national or regional borders. Our Secure Research Environment and Lifebit Trusted Research Environment are built to solve these exact problems.
3. Future-Proofing Your Research Portal
A portal built today must be ready for tomorrow’s data and analytical challenges. Key considerations include:
- Scalability: Portals must be built on cloud-native infrastructures that can scale on demand to handle petabyte-scale data volumes and computationally intensive analyses.
- Multi-omics Integration: The future is about weaving together genomics, proteomics, metabolomics, transcriptomics, and clinical data into a single, comprehensive biological picture of a patient.
- Real-World Evidence Generation: Integrating data from everyday clinical practice—like EHRs, claims data, and patient registries—is crucial for understanding real-world treatment effectiveness and safety.
- Patient-Generated Health Data (PGHD): A rapidly growing component of RWE, this includes data from consumer wearables (e.g., smartwatches tracking heart rate and activity), mobile health apps, and digital patient-reported outcome surveys. Portals must be equipped to ingest, standardize, and analyze this continuous stream of data to capture a more complete view of a patient’s health journey outside of the clinic.
- Pharmacovigilance: AI-driven analytics can proactively monitor aggregated real-world data for faint signals of adverse drug events, enabling near real-time drug safety surveillance—a potentially life-saving capability.
Your Next Step: Harness the Power of Connected Data
We’ve seen how clinical data portals are breaking down silos to create a triple win for research, clinical care, and patients. While challenges in data privacy, security, and standardization are significant, they are being met with innovative solutions like federated analysis and secure, compliant frameworks. Our approach to Clinical Data Governance reflects this commitment.
The future is already here. AI and machine learning are open uping new insights, and federated analysis is enabling global collaboration without compromising patient privacy. This is the foundation of our Trusted Research Environments. The era of isolated data is ending, replaced by interconnected highways of knowledge.
This shift promises faster trials, better treatments, and more personalized medicine. The power of connected data isn’t just about what we can find—it’s about how quickly we can translate those findies into better health for everyone.
For organizations ready to build next-generation data solutions, exploring a Lifebit Federated Biomedical Data Platform provides a proven, secure, and scalable path forward.


