Recruiting in the Digital Age: Smart Strategies for Clinical Trials

Clinical Trial Digital Patient Recruitment: Top 5 for 2025
The Race Against Time in Clinical Research
Clinical trial digital patient recruitment has become the critical bottleneck that can make or break modern medical research. Here’s what you need to know:
Key Digital Recruitment Strategies:
- Targeted digital outreach through social media and search platforms
- EHR data mining with AI-powered patient matching algorithms
- Patient portals and online communities for direct engagement
- E-consent and remote screening to reduce barriers
- Real-time analytics to optimize recruitment performance
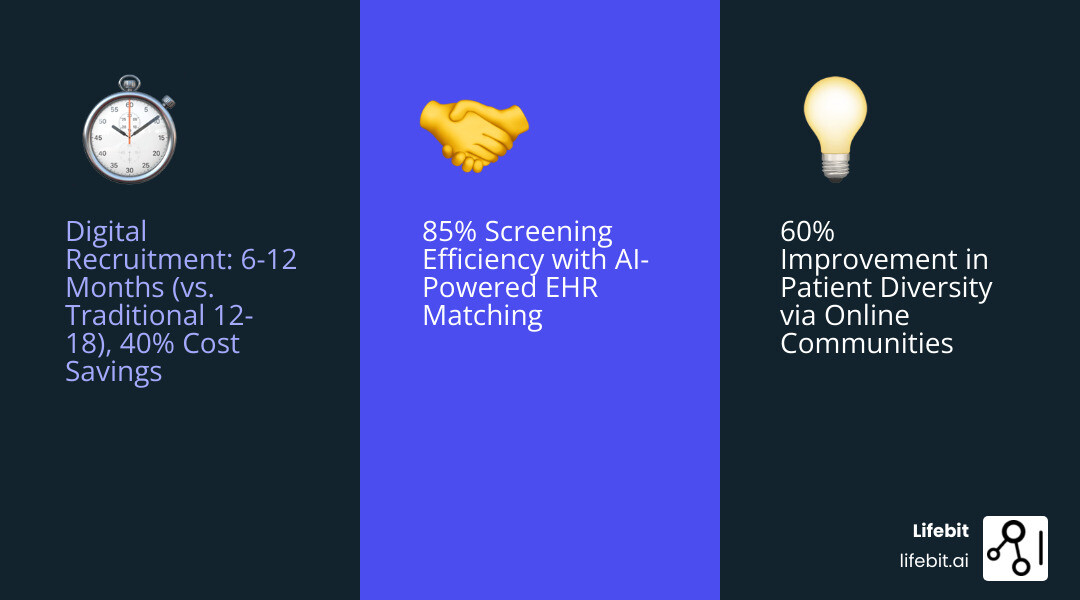
The numbers tell a stark story. 80% of clinical studies fail to meet their enrollment deadlines, while recruitment costs consume 40% of all trial expenditures. For many sponsors, every month of delay costs an additional $1 million. The financial stakes are enormous – a failed clinical trial can cost between $800 million to $1.4 billion.
Traditional recruitment methods – print ads, physician referrals, and site-based approaches – simply can’t keep pace with today’s complex protocols and faster research timelines. Meanwhile, 4.54 billion internet users worldwide represent an untapped pool of potential participants who are actively seeking health information online.
The shift to digital isn’t just about speed and cost. It’s about reaching diverse patient populations, improving data quality, and building more inclusive trials. Social media recruitment, machine learning algorithms, and patient-centric platforms are changing how we connect patients with potentially life-saving treatments.
I’m Maria Chatzou Dunford, CEO of Lifebit, where I’ve spent over 15 years developing AI-powered platforms that enable secure, federated analysis of biomedical data for clinical trial digital patient recruitment. My work focuses on breaking down data silos to accelerate patient matching and improve trial outcomes through cutting-edge technology.
Simple clinical trial digital patient recruitment word guide:
- clinical trial recruitment strategies
- clinical trial recruitment digital results
- clinical trial recruitment digital case study
The High Cost of Outdated Recruitment: Why Traditional Methods Fall Short
Let’s be honest – traditional patient recruitment feels like trying to find someone in a crowded stadium by shouting their name. It’s slow, expensive, and you’re probably not reaching the right people anyway.
The biggest problem? Geographic limitations that trap trials in a tiny bubble. When recruitment relies on local clinics and physician referrals, the participant pool is restricted to those near a study site. This model is fundamentally broken for modern research, especially for rare diseases or trials requiring specific genetic markers, where eligible patients are scattered globally. Relying on site-based recruitment in this context is like trying to find a needle in a nationwide haystack by only looking in your backyard. It’s no wonder many rare disease trials struggle for years to find patients.
This creates a domino effect of low patient awareness. Most patients have never had a conversation with their doctor about clinical research as a care option. Studies show that while patients are willing to participate, the topic is rarely broached, creating a massive information gap. Millions of potential participants simply don’t know trials exist or how they could benefit. Traditional methods are too passive to bridge this gap, leaving countless eligible patients in the dark. It’s no wonder that a third of all delays for Phase III studies are caused by difficulties finding patients to take part.
Meanwhile, study sites are drowning under the high site burden of traditional recruitment. Clinical research coordinators, who should focus on patient care, are forced to become part-time marketers and administrators. Their days are consumed by manually sifting through charts, playing phone tag with candidates, and managing paper consent forms. This isn’t just inefficient; it leads to burnout, errors, and takes valuable time away from enrolled patients. It’s like asking your chef to also handle restaurant marketing – something’s bound to get burned!
The diversity problem is equally troubling. When recruitment stays within specific geographic areas or physician networks, we miss entire communities. This matters deeply because clinical trials need to reflect real-world patient populations to ensure treatments work safely for everyone. Currently, less than 5% of cancer patients enroll in clinical trials, highlighting just how many people we’re failing to reach.
Then there’s the inefficient screening process that makes everything worse, creating a notoriously ‘leaky’ recruitment funnel. The ‘screen failure rate’—the percentage of screened patients found to be ineligible—can exceed 80% for complex trials. Each failure represents wasted time and resources. Manual chart reviews, multiple required in-person visits, and mountains of paperwork create a slow, costly gauntlet that discourages many promising candidates. Every step adds delays and expenses.
The numbers don’t lie about these traditional bottlenecks. Recruitment costs make up 40% of trial expenditures, while 80% of studies fail to meet enrollment deadlines. For sponsors, every delayed month costs an additional $1 million. When trials fail completely, the price tag can reach $800 million to $1.4 billion.
These aren’t just statistics – they represent delayed treatments, missed opportunities, and patients who could benefit from life-changing therapies but never get the chance. Clinical trial digital patient recruitment offers a way out of this expensive, inefficient maze.
The solution isn’t tweaking the old system – it’s embracing entirely new approaches that can reach patients where they are, when they’re ready. You can find more info about Clinical Trial Recruitment Strategies to see how digital methods are changing this landscape.
Core Strategies for Clinical Trial Digital Patient Recruitment
Here’s where the real change happens. Clinical trial digital patient recruitment isn’t just about swapping newspaper ads for Facebook posts – it’s about fundamentally reimagining how we connect with patients. Think of it as moving from fishing with a single hook to using a smart net that knows exactly what fish you’re looking for.
The beauty of digital recruitment lies in its ability to be both broad and precise. We can reach millions of people while still targeting those most likely to benefit from our research. It’s like having a conversation with thousands of patients simultaneously, but making each one feel personal and relevant.
Using Digital Channels for Patient Engagement
The digital world offers us incredible opportunities to meet patients where they already are – online, searching for answers, and connecting with others who share their health journey.
Targeted digital outreach transforms how we find the right participants. Instead of hoping the right person sees a generic flyer, we can use sophisticated targeting on platforms like Facebook, Twitter, and Instagram to reach people based on their demographics, interests, and health-related behaviors. It’s remarkably effective – we’re essentially raising our hand to people who are already looking for solutions.
Patient communities represent some of the most valuable real estate in digital recruitment. Platforms like HealthOpen uped host vibrant communities for hundreds of conditions, where patients actively share experiences and seek support. These aren’t just random internet forums – they’re trusted spaces where people discuss their most personal health challenges. When we engage respectfully in these communities, we’re not interrupting their day; we’re offering hope.
The key is creating content that actually helps people. Through blogs, videos, and infographics, we educate potential participants about their condition, treatment options, and how clinical trials work. This builds genuine trust rather than just pushing enrollment numbers. When someone understands why a trial matters and how it could help them, they’re far more likely to participate meaningfully.
Search engine visibility ensures we’re there when patients need us most. When someone types “new treatments for diabetes” or “clinical trials near me” at 2 AM (and trust me, they do), our trials should appear. With 80% of internet users actively seeking healthcare information online and 62% of adults with chronic conditions relying primarily on digital channels, this isn’t optional – it’s essential.
For deeper insights into these strategies, check out our comprehensive guide on digital clinical trial recruitment.
Leveraging EHRs and Real-World Data for Precision Targeting
While digital outreach casts a wide net, Electronic Health Records (EHRs) and Real World Data let us fish with precision. This is where the magic of modern technology really shines.
EHRs contain treasure troves of patient information – diagnoses, treatments, lab results, medication histories. By securely analyzing this data, we can identify patients who match specific trial criteria before they even know the trial exists. It’s like having a crystal ball that shows us exactly which patients could benefit from our research.
Machine learning algorithms take this a step further. These intelligent systems can analyze vast datasets to identify patterns that human reviewers might miss. They can predict which patients are most likely to be eligible, which ones might face barriers to participation, and even which recruitment messages might resonate best. The algorithms get smarter with each dataset they analyze, constantly improving their accuracy.
AI-powered pre-screening automates much of the tedious initial screening work. Natural language processing can extract relevant information from unstructured medical notes, while intelligent chatbots can ask initial screening questions 24/7. This means potential participants can start their journey immediately, rather than waiting for business hours.
Data mining helps us find hidden patient populations within large healthcare systems. We might find clusters of patients with rare conditions who would never have been identified through traditional methods, or uncover geographic patterns that inform our recruitment strategy.
This precision targeting dramatically reduces screen-fail rates and speeds up enrollment timelines. Instead of screening 100 patients to find 10 eligible ones, we might screen 20 to find 15. The efficiency gains are remarkable. Learn more about how AI transforms clinical research in our article on AI for clinical trials.
Engaging Patients Through Portals and Online Communities
Once we’ve captured a patient’s interest, the real work begins. This is where clinical trial digital patient recruitment becomes about relationship building, not just lead generation.
Patient portals create secure, convenient spaces for ongoing communication. Think of them as digital waiting rooms where patients can access study information, complete questionnaires, and even sign consent forms from their kitchen table. This convenience removes many traditional barriers – no more taking time off work for initial screening visits or playing phone tag with study coordinators.
Established online health communities offer incredible opportunities for authentic engagement. Carenity, for example, supports 500,000 patients and caregivers across 1,200 chronic and rare diseases. These platforms already have the trust and attention of patients – we just need to provide genuine value to their communities.
Email marketing keeps potential participants engaged throughout the often lengthy recruitment process. Well-crafted email sequences can educate, update, and encourage patients while they’re deciding whether to participate. The key is providing real value – study updates, educational content, or connections to support resources – rather than just pushing for enrollment.
Advocacy group partnerships amplify our reach exponentially. When a trusted patient organization endorses a trial, it carries far more weight than any advertisement we could create. These groups understand their members’ needs intimately and can help us communicate in ways that truly resonate.
The goal is creating a seamless, supportive experience that makes participation feel like a natural next step rather than a leap of faith. When patients feel informed, supported, and valued throughout the process, they’re more likely to enroll and stay engaged through completion.
For more details on how we improve trial matching through these digital strategies, explore our insights on enhancing clinical trial matching.
Navigating the Challenges of Digital Recruitment
While clinical trial digital patient recruitment offers immense advantages, it’s not without its problems. Like any powerful tool, it demands careful handling, especially concerning data privacy, security, and ensuring equitable access. We’ve learned that addressing these challenges head-on is paramount for successful and ethical digital recruitment.
Ensuring Data Privacy and Regulatory Compliance in Clinical Trial Digital Patient Recruitment
The digital landscape, while expansive, is also a minefield of potential privacy breaches if not steerd correctly. Handling sensitive patient health information (PHI) requires an unwavering commitment to data security and strict adherence to regulatory frameworks.

- Data security and patient privacy: With the increasing reliance on digital platforms, it is imperative to prioritize data privacy and security. Patient data must be handled with the utmost care, ensuring compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the US and the General Data Protection Regulation (GDPR) in Europe. Implementing robust security measures, including encryption, access controls, and regular audits, is non-negotiable. We understand that trust is built on transparency and security, especially when dealing with personal health data.
- Informed e-consent: Digital recruitment often involves electronic consent (e-consent). While e-consent offers convenience and efficiency, it must uphold the same rigorous standards as traditional paper consent. This means ensuring participants fully understand the trial, their rights, and how their data will be used. Multimedia elements, interactive modules, and clear, concise language can improve comprehension. The research indicates that e-consenting appears to have been used effectively for several years, primarily because extensive guidance regarding its use has been issued by regulatory bodies. This relative clarity is a stark contrast to the broader digital recruitment landscape.
One of the ongoing challenges is the lack of comprehensive regulatory guidance specifically for the wider use of digital technologies in clinical trial digital patient recruitment. This regulatory uncertainty can make sponsors and sites hesitant to adopt novel tools, despite their potential benefits. We advocate for clearer frameworks that balance innovation with patient protection. Our work in Trusted Research Environments (TREs) directly addresses these concerns by providing secure, compliant platforms for data analysis, ensuring patient data remains protected while enabling groundbreaking research.
Improving Diversity and Overcoming the Digital Divide
Beyond privacy, digital recruitment faces the crucial task of fostering diversity and inclusion, while also bridging the infamous “digital divide.”
- Health equity and inclusive outreach: Clinical trials have historically struggled with diversity, leading to findings that may not be fully generalizable across all populations. For instance, less than 5% of cancer patients are enrolled in clinical trials. Furthermore, despite representing ~13% of the US population, Black communities only accounted for ~3% of COVID-19 vaccine trial participants, while unfortunately experiencing ~21% of COVID-19 mortality. This disparity is unacceptable. Digital technologies offer powerful tools to address this by enabling highly targeted and culturally sensitive outreach.
- Culturally targeted content: It’s not enough to just reach diverse populations; we must speak to them in a way that resonates. This means creating culturally custom educational materials, using trusted community voices, and employing diverse imagery in our digital campaigns. Culturally custom multimedia can significantly improve the intent to participate among underrepresented groups, demonstrating that attitudinal barriers can be overcome with thoughtful content.
- Technological barriers and the digital divide: While billions are online, access is not universal or uniform. The “digital divide” refers to the gap between those who have ready access to the internet and digital devices and those who do not. This can include individuals in rural areas, older adults, low-income communities, or those with limited digital literacy. A systematic review on digital technologies and trial diversity noted that digital recruitment may inadvertently exacerbate the digital divide unless disparities in access and literacy are explicitly addressed. To counter this, we must:
- Offer multiple digital pathways for engagement (e.g., mobile-friendly websites, text messaging, email, phone lines).
- Provide support for those with limited digital literacy.
- Consider hybrid approaches that combine digital outreach with community-based, in-person engagement where necessary.
- Design digital tools with accessibility in mind, ensuring they are user-friendly for all.
Leveraging digital technologies to improve diversity and inclusion in clinical trial recruitment is not just an ethical imperative; it’s a scientific necessity. It ensures that new treatments are safe and effective for everyone, not just a select few. Our commitment to Innovations in Clinical Trial Recruitment includes a strong focus on making trials more equitable and representative.
Measuring Success and Embracing the Future
Here’s the truth: launching a clinical trial digital patient recruitment campaign is just the beginning. The real magic happens when we dive deep into the data, measure what’s working, and continuously refine our approach. It’s like tending a garden – you can’t just plant seeds and walk away. You need to monitor growth, adjust watering, and prune what isn’t thriving.
Key Metrics for Effective Clinical Trial Digital Patient Recruitment
Think of metrics as your recruitment compass. Without them, you’re essentially flying blind, hoping your efforts will somehow lead to successful enrollment. But with the right Key Performance Indicators (KPIs), you can steer with confidence and make data-driven decisions that actually move the needle.
Cost per enrolled participant is often our north star metric. It tells us exactly how much we’re spending to get one person successfully enrolled. However, this metric must be viewed with nuance; a low cost is meaningless if the cohort lacks diversity or has poor retention. An effective strategy balances cost with participant quality. What we’ve found through our research is fascinating – optimized email and targeted electronic outreach often outperform paid social ads in cost per enrollment. It’s not always about the flashiest platform; sometimes the most direct approach wins.
Conversion rates paint the full picture of our recruitment funnel. We track every step: how many ad impressions turn into clicks, how many clicks become pre-screens, and how many of those actually enroll. Tracking these micro-conversions reveals bottlenecks, such as a confusing landing page or a lengthy form, allowing us to fix them and improve overall efficiency.
Screen Failure Rate is the percentage of potential participants who enter screening but are found to be ineligible. A high rate wastes site resources and disappoints patients. Digital recruitment aims to lower this rate by using precise targeting and detailed online pre-screeners to better qualify candidates before they reach the site.
Patient Retention Rate measures the percentage of enrolled participants who complete the study, which is essential for a trial’s validity. Digital tools like patient portals and telehealth check-ins keep patients engaged and feeling valued, which can significantly improve retention.
Enrollment velocity keeps us honest about our timelines. We track key milestones like Time to First Patient In (FPI) and forecast the Time to Last Patient In (LPI). Missing these deadlines has cascading financial consequences, and since 80% of studies fail to meet enrollment deadlines, staying on top of this pace is crucial.
Channel ROI shows us where to double down and where to pull back. Maybe our Facebook campaigns are crushing it while our Google ads are falling flat. This insight lets us reallocate budget to the channels that actually deliver results.
But here’s what many people miss: participant diversity and representation metrics matter just as much as enrollment numbers. We’re not just filling seats; we’re building trials that reflect the real world. This means tracking whether we’re reaching underrepresented communities and ensuring our digital strategies don’t accidentally exclude anyone.
The beauty of digital recruitment is that we can pivot quickly. When we spot a trend in our data, we can adjust campaigns in real-time, test new approaches, and optimize as we go. For more insights on tracking success, check out our detailed analysis on More info on Digital Recruitment Results.
Future Trends: AI, Decentralization, and Federated Learning
The future of clinical trial digital patient recruitment isn’t just bright – it’s absolutely revolutionary. We’re standing at the edge of changes that will make today’s digital recruitment look like the early days of dial-up internet compared to fiber optic broadband.

Artificial intelligence is evolving beyond simple patient matching. Imagine AI-powered chatbots that can handle initial patient screening with the warmth and understanding of a human coordinator. Picture predictive analytics that can forecast enrollment challenges months before they happen. We’re talking about AI that creates personalized outreach content for each potential participant, making every interaction feel genuinely relevant.
Decentralized Clinical Trials are flipping the traditional model on its head. Instead of asking patients to travel to research sites, we’re bringing the trial to them. Wearable technology quietly collects vital signs and activity data while participants go about their daily lives. Telehealth connects patients with investigators through video calls from their living rooms. This isn’t just convenient – it’s transformative for people who live hours away from research centers or have mobility challenges.
What’s particularly exciting is how leveraging remote technology and decentralization tools may increase patient consent rates. When participating in a trial becomes easier and less disruptive, more people say yes. The presence of e-consent tools may lead to better enrollment because patients can review materials at their own pace, in their own space, without feeling rushed.
Federated Learning might sound technical, but it’s solving a very human problem. This approach lets us train machine learning models on data from multiple hospitals and research institutions without the data ever leaving its original location. It’s like having a brilliant detective who can solve cases by examining clues from multiple crime scenes simultaneously, but never actually visiting any of them. This maintains strict privacy while open uping the power of vast, diverse datasets for better patient identification.
At Lifebit, we’re not just observing these trends – we’re building them into reality through our unified platform. Our platform’s key components include:
- Trusted Research Environment (TRE): Ensures secure, compliant analysis of sensitive patient data.
- Trusted Data Lakehouse (TDL): Manages diverse biomedical information, making it readily available when researchers need it.
- R.E.A.L. (Real-time Evidence & Analytics Layer): Provides real-time insights and AI-driven safety surveillance, crucial for monitoring recruitment progress and patient safety.
These aren’t separate tools working in isolation. They’re integrated components that create a federated governance system, enabling secure collaboration across different data sources while maintaining privacy. We’re empowering large-scale, compliant research across biopharma, governments, and public health agencies.
The future we’re building isn’t just about recruiting patients faster or cheaper. It’s about creating a smarter, more connected ecosystem where medical research can flourish while keeping patient privacy and safety at the center of everything we do. Learn more about our approach to Decentralized Clinical Trial Model and explore our Federated Trusted Research Environment to see how we’re making this future a reality today.
Conclusion: Building Faster, Smarter, and More Inclusive Trials
We’ve reached a turning point in medical research. Clinical trial digital patient recruitment has evolved from a nice-to-have innovation into an absolute necessity for successful studies. The old ways of finding patients through newspaper ads and physician referrals simply can’t keep up with today’s complex trials and tight timelines.
Think about what we’ve finded together. Traditional recruitment methods were costing us dearly – with 80% of studies missing their enrollment deadlines and recruitment eating up 40% of total trial budgets. Every delayed month costs sponsors around $1 million. Those numbers aren’t just statistics; they represent delayed treatments for patients who desperately need them.
But here’s the exciting part: digital strategies are changing everything. We can now reach patients where they already spend their time – online, in patient communities, and through targeted social media campaigns. Machine learning algorithms can scan through thousands of electronic health records to find the perfect candidates in minutes, not months. Patient portals make participation easier and less intimidating for everyone involved.
The real magic happens when we combine speed with inclusivity. Digital recruitment doesn’t just fill trials faster; it helps us build more diverse and representative study populations. When we use culturally targeted content and overcome technological barriers, we’re not just checking diversity boxes – we’re ensuring that new treatments work for everyone, not just a select few.
Patient-centricity isn’t just a buzzword anymore. It’s the foundation of modern recruitment. When we bring trials to patients through decentralized models, telehealth visits, and wearable technology, we’re removing the biggest barriers to participation. No more taking time off work for multiple site visits or traveling hundreds of miles to participate in potentially life-saving research.
The role of secure data platforms cannot be overstated. Privacy and compliance aren’t obstacles to innovation – they’re the bedrock that makes digital recruitment trustworthy. Patients need to know their data is safe, and researchers need platforms that can handle sensitive health information while still enabling groundbreaking findies.
At Lifebit, we’ve built our federated AI platform specifically to address these challenges. Our technology securely connects global biomedical data while keeping it exactly where it belongs – protected within trusted environments. We’re not just talking about faster recruitment; we’re enabling real-time insights, advanced analytics, and secure collaboration across entire research ecosystems.
The future is already here. Artificial intelligence, federated learning, and decentralized trials aren’t science fiction – they’re tools we can use today to transform how we conduct research. Every successful digital recruitment campaign brings us closer to a world where participating in clinical trials is accessible, inclusive, and genuinely patient-friendly.
Ready to leave outdated recruitment methods behind? Learn how to accelerate your research with our unified platform and join the digital revolution that’s making clinical trials faster, smarter, and more inclusive for everyone.


