The Future of Trials: Comprehensive Clinical Trial Solutions

Clinical trial solution: Unify for 2025
Why Clinical Trial Solutions Are Changing Modern Research
A clinical trial solution marks a shift from fragmented, inefficient research systems to unified platforms that streamline clinical development. These solutions tackle today’s biggest trial challenges: over 80% face enrollment delays, manual processes cost sponsors over $1 billion annually, and less than 3% of eligible patients participate due to high perceived burden.
Key components of modern clinical trial solutions include:
- Unified patient experience: eConsent, eCOA/ePRO, telehealth, and remote monitoring in one platform.
- Streamlined site operations: Single sign-on, automated workflows, and real-time dashboards.
- Harmonized data management: End-to-end traceability, AI-powered analytics, and regulatory-ready data.
- Flexible trial models: Support for on-site, hybrid, and fully decentralized studies.
- Advanced analytics: Real-time risk monitoring, predictive recruitment, and operational insights.
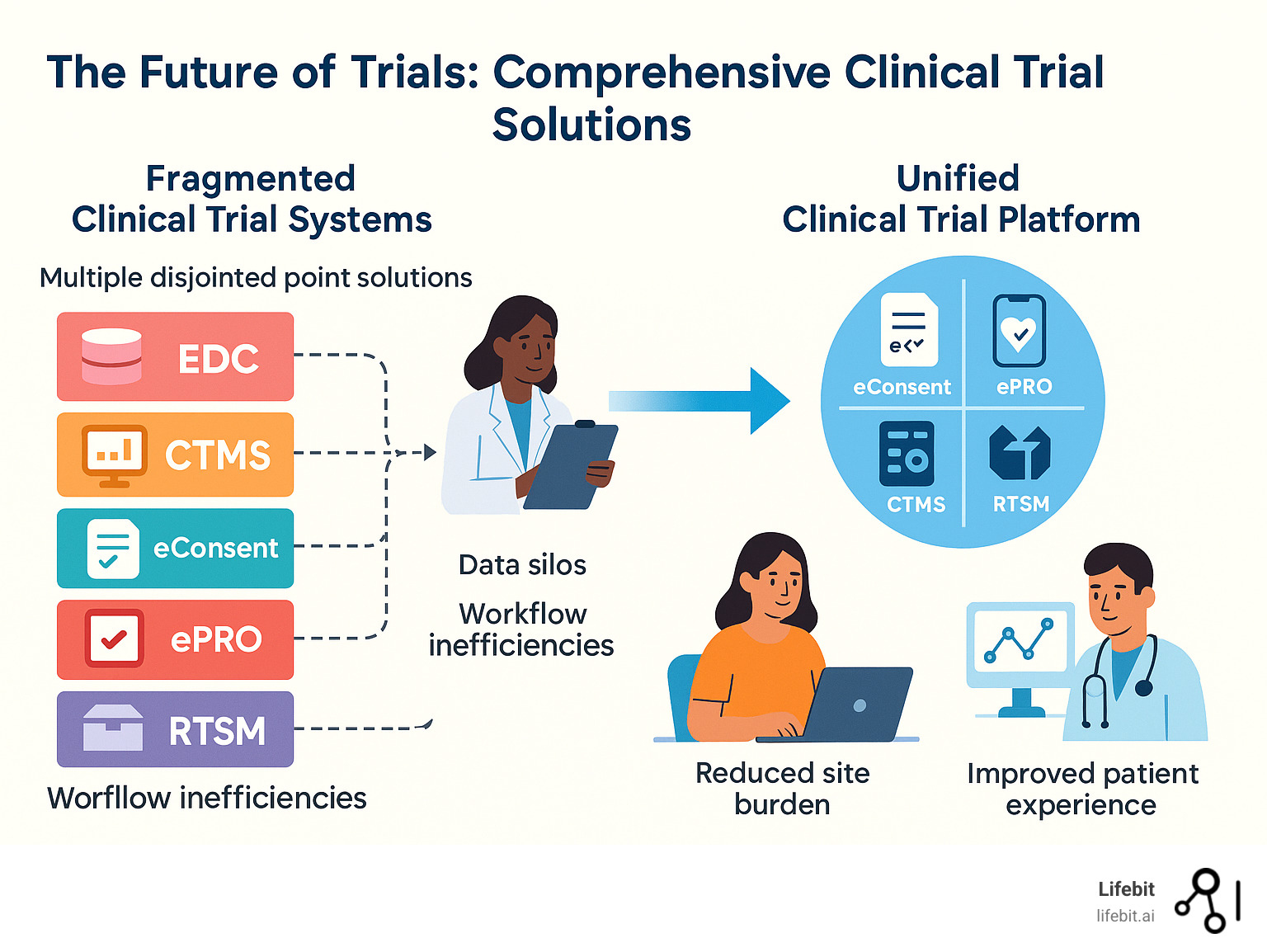
The traditional approach of using multiple point solutions creates data silos, increases site burden, and leads to poor patient experiences. Modern unified platforms deliver on the promise to build a study once, enter data once, and do everything in one place.
The results are compelling. Organizations using comprehensive clinical trial solutions report 6 months faster time-to-market, 60% fewer clinic visits, and over $25 million in savings per trial. These platforms support 82 languages and manage over 1.5 million participants worldwide.
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve seen how the right clinical trial solution can transform research. My 15 years in computational biology and biomedical data have shown me that federated, AI-native platforms are essential for the future of clinical development.
Handy clinical trial solution terms:
- clinical trial recruitment strategies
- digital clinical trial recruitment
- clinical trial recruitment digital case study
The Problem with Fragmented Systems: Why Today’s Trials Are Inefficient
Today’s clinical trial landscape is often a fragmented reality, costing the industry dearly. The root of the problem lies in siloed data sources. When an Electronic Data Capture (EDC) system doesn’t communicate with a Clinical Trial Management System (CTMS) or randomization platform, data becomes trapped. This necessitates manual processes to move information, creating a massive financial drain.
Large pharmaceutical companies face over $1 billion in annual revenue loss from these manual processes alone. Copying and reconciling data across platforms inevitably leads to data quality issues. For example, a simple transcription error when moving lab results from a LIMS report into an EDC can lead to incorrect safety assessments or flawed efficacy analysis. These errors multiply, compromising the accuracy that trials demand. Without a unified view, predictability becomes a pipe dream, making it impossible to get real-time insights into enrollment, identify risks early, or forecast milestones.
This fragmentation places an immense burden on all stakeholders. For sponsors and CROs, it creates operational bottlenecks and delayed timelines. Inefficient oversight and complex data aggregation mean problems fester, and the financial impact of delaying a drug’s market entry can be millions per day. Compliance risks also multiply. When audit trails are scattered across multiple vendors, demonstrating adherence to Good Clinical Practice (GCP) during an FDA or EMA inspection becomes a nightmare. Reconstructing the full lifecycle of a data point—from source to submission—is nearly impossible, increasing the risk of regulatory findings like Form 483s.
Clinical sites bear the heaviest burden, facing administrative overload from juggling multiple system logins and performing repetitive data entry. Staff spend valuable time in training for disparate systems instead of focusing on patient care, leading to staff burnout and high turnover rates. This friction is a major contributor to why less than 3% of eligible patients participate in trials.
From the patient’s perspective, this creates a confusing and burdensome experience. They are asked to use one app for diaries, another for appointments, and receive paper forms for consent. This cognitive load, combined with a lack of transparency and inconvenient travel for routine assessments, leads to poor retention rates. A staggering less than 10% of clinical trial data is collected away from sites, meaning patients still make frequent trips for assessments that could be done remotely with the right clinical trial solution.
Modern unified solutions are designed to solve these problems by bringing the entire research experience into a single, efficient, and patient-friendly platform.
The Rise of the Unified Clinical Trial Solution
The industry is breaking free from fragmented systems with the rise of unified clinical trial solutions that bring everything under one roof. These platforms create end-to-end workflows in a single, cohesive environment, establishing a single source of truth for all trial information. This eliminates the need to reconcile conflicting data from different systems.
The real change comes from adding AI and automation. These tools dramatically improve efficiency and data quality by automating tasks that once required hours of manual work, freeing teams to focus on research and patient care.
| Feature | Point Solutions | Unified Platform |
|---|---|---|
| Data Flow | Siloed, manual transfers, reconciliation needed | Seamless, real-time integration, single source of truth |
| Login Burden | Multiple logins, different credentials | Single Sign-On (SSO), reduced login fatigue |
| Workflows | Disconnected, prone to manual errors | Automated, integrated, end-to-end |
| Data Quality | Higher risk of errors, inconsistencies | Improved, automated validation, provenance tracking |
| Oversight | Fragmented, delayed insights | Real-time, comprehensive dashboards |
| Patient Exp. | Complex, burdensome, inconsistent | Simplified, patient-centric, consistent |
| Cost | Higher TCO, integration costs | Reduced TCO, streamlined vendor management |
A Patient-First Approach to a modern clinical trial solution
To increase patient participation, the trial experience must be improved. The days of navigating multiple confusing systems and traveling for routine check-ups are over.

A patient-first approach uses a single, familiar interface for all interactions. This includes:
- Dynamic eConsent: This goes beyond a digital signature. It uses interactive multimedia content, such as videos and diagrams, to explain complex trial procedures. Embedded comprehension quizzes ensure patients truly understand what they are agreeing to, strengthening the informed consent process and reducing early dropouts.
- Engaging eCOA/ePRO: Electronic Clinical Outcome Assessments (eCOA) and Patient-Reported Outcomes (ePRO) capture data directly from the patient. This eliminates recall bias associated with paper diaries and improves compliance through automated reminders. The data is higher quality, entered in real-time, and can trigger alerts to the clinical site if a patient reports a concerning symptom.
- Integrated Telehealth: Instead of traveling for a routine follow-up, patients can connect with their study team via secure video calls within the same platform, saving time and money.
Remote Patient Monitoring devices automatically send health data, while coordinated home visits handle in-person needs. This model allows up to 74% of assessments to be conducted at home, putting patients first. The results are clear: organizations using these platforms report over 60% improvement in patient experience scores, leading to better recruitment and retention. Accessibility is also key, with top solutions supporting up to 82 languages and offline functionality.
Empowering Sites and Streamlining Operations
Unified platforms give clinical sites their time back. Single Sign-On eliminates the frustration of multiple logins, providing one-click access to all necessary tools. This includes integrated CTMS capabilities, eISF/eSource tools, and automated scheduling that aligns with protocol-defined visit windows. The platform automates burdensome administrative tasks, such as tracking and disbursing patient stipends, managing real-time training records for staff, and simplifying complex financial budgeting and payments. By automating these workflows, the platform allows site staff to focus on what matters most: patient care and high-quality data collection.
Unifying Data for Deeper Insights and a predictable clinical trial solution
A unified clinical trial solution makes data work harder. It seamlessly integrates information from EDC systems, eCOA responses, medical imaging, lab results, wearable sensors, and Real-World Data. This creates a rich, comprehensive picture that is impossible to achieve with fragmented systems. AI-driven recruitment can analyze this data to identify suitable patients with unprecedented precision. For example, AI algorithms can scan millions of de-identified EMRs to find patients matching complex criteria—like a specific genetic mutation and a history of failing two prior lines of therapy—that would be impossible for a human to find manually. This not only accelerates enrollment but also helps ensure a diverse and representative patient population.
Data provenance and audit trails are built-in, ensuring full traceability. As noted, Big data analysis should not be a big burden, and the right platform makes complex analytics intuitive. Baked-in compliance frameworks (GCP, 21 CFR Part 11, GDPR, HIPAA) and support for industry standards (CDISC, HL7/FHIR) ensure data integrity. Real-time risk-based monitoring and pharmacovigilance workflows provide early warnings and ensure safety events are handled properly, leading to predictable timelines and cleaner data.
Supporting Modern Trial Models and Therapeutic Areas
A comprehensive clinical trial solution must be adaptable and versatile, ready to handle any research challenge. This flexibility is what makes a platform valuable both today and in the future.

Modern unified platforms are built with adaptability to support any research model, from traditional site-based studies to hybrid approaches and fully decentralized trials. This scalability allows a study to grow from 50 patients to thousands without switching systems, future-proofing research by building on a foundation that grows with your ambitions.
From On-Site to Decentralized: A Flexible Approach to Trial Design
The pandemic accelerated the shift in how we think about clinical trials. A modern clinical trial solution supports this by seamlessly enabling hybrid trials that blend in-person and remote care. Fully decentralized trials (DCTs) take this further, leveraging the fact that 74% of assessments can be conducted at home.
The platform makes this possible through integrated telehealth, remote monitoring, and intelligent logistics management. This is a critical component, as the platform must orchestrate a complex supply chain that includes direct-to-patient shipment of investigational products (often temperature-sensitive), delivery of home-health nursing kits, and coordination of specialty couriers for biological sample collection. The system provides end-to-end sample tracking with a clear chain of custody, ensuring a blood sample drawn in a patient’s home is properly handled, transported, and analyzed at a central lab. Remote monitoring gives sponsors real-time visibility without constant site visits, providing richer, more continuous data from patients in their everyday environments. The platform also maintains robust on-site support for assessments that require clinical oversight, offering the flexibility to choose the right approach for each situation.
Precision and Power in Complex Therapeutic Areas
Specialized therapeutic areas like oncology, cardiovascular research, and rare diseases demand advanced capabilities. Biomarker-driven trials in these fields require sophisticated data management that modern platforms provide.
- Oncology: Modern oncology trials often use adaptive designs, where cohorts are expanded or closed based on real-time biomarker data. A unified platform is essential to integrate complex data types—such as NGS data for patient stratification, imaging data for tumor response (e.g., RECIST 1.1 criteria), and circulating tumor DNA (ctDNA) levels—to enable rapid decision-making.
- Rare Diseases: For these conditions, patients are few and geographically scattered. A unified platform with strong DCT capabilities is often the only feasible way to conduct a trial, removing the immense travel burden that would otherwise make participation impossible.
- Cardiovascular: These trials increasingly rely on digital endpoints from wearables, such as continuous ECG monitoring for arrhythmia detection or actigraphy data for measuring physical activity. The platform must not only ingest this high-frequency data but also guide device onboarding and validation to ensure the data is of regulatory-grade quality.
These platforms excel at genomics data management, handling massive datasets from NGS pipelines. For complex genomic analyses, platforms like the precisionFDA Platform show the level of secure, compliant computational power required. A robust clinical trial solution can also bridge translational research to clinical use, allowing research pipelines to be validated and deployed in GxP-compliant environments, accelerating the entire drug development process.
Measuring Success: The Tangible ROI of a Unified Platform
The shift to a unified clinical trial solution delivers a measurable, bottom-line impact. Modern platforms provide comprehensive operational analytics and Key Performance Indicators (KPIs) through real-time dashboards. This allows sponsors to move beyond lagging indicators and proactively manage trial performance by tracking metrics like:
- Enrollment Velocity: Tracking actual vs. projected enrollment rates per site and country.
- Screen Failure Rate: Identifying sites or regions where eligibility criteria are causing bottlenecks.
- Data Quality & Query Rates: Monitoring data cleanliness in real time, measuring the time from data entry to query and from query to resolution.
- Protocol Deviations: Automatically flagging deviations to enable immediate corrective action.
This data-driven oversight enables decisions that accelerate research. The time savings are remarkable, with unified platforms delivering a 6-month acceleration in time-to-market. This translates directly to millions in additional revenue and extended patent protection.
The financial benefits are significant. In cardiovascular and metabolic studies, organizations report 60% fewer in-person clinic visits, 43% lower cost-per-patient, and over $25 million in savings per trial. These savings are derived from:
- Reduced Site Monitoring Costs: With remote monitoring and centralized data review, the need for expensive on-site visits by Clinical Research Associates (CRAs) is drastically reduced.
- Lower Administrative Overhead: Consolidating multiple vendor contracts into a single platform reduces legal, procurement, and management costs.
- Fewer Protocol Amendments: Real-time data insights allow for early detection of issues, preventing the need for costly and time-consuming protocol amendments down the line.
In respiratory studies, remote measurements have helped reduce required patient numbers by 50% while finishing 6 months ahead of schedule. For rare disease research, where travel is a major burden, these platforms have reduced site visits by up to 40%, making participation possible for more patients.
Regulatory benefits are also substantial. Centralizing data, audit trails, and documentation in one platform streamlines inspections. Instead of scrambling to pull records from a dozen systems, sponsors can provide an inspector with secure, read-only access to a single source of truth where every action is time-stamped and attributable. This accelerates regulatory submissions by ensuring data is clean, complete, and available in submission-ready formats like SDTM. From a total cost of ownership (TCO) perspective, managing a single platform provides significant administrative savings. The ultimate ROI comes from predictability—the ability to anticipate challenges and adjust on the fly, fundamentally changing how quickly life-saving treatments reach patients.
Frequently Asked Questions about Clinical Trial Solutions
When moving to a unified clinical trial solution, sponsors, sites, and researchers often have questions about how these platforms handle the complexities of modern research. Here are the most common concerns.
How is patient data privacy and security ensured in a unified platform?
Patient trust is paramount, so robust data privacy and security are core to any modern clinical trial solution. At Lifebit, our platform is built on comprehensive governance frameworks that adhere to the gold standards of clinical research: GCP (Good Clinical Practice), 21 CFR Part 11, and 21 CFR Part 820. We also ensure full compliance with GDPR and HIPAA.
Technical safeguards include end-to-end data encryption (both at rest and in transit) and granular role-based access control to ensure users only see authorized data. We employ sophisticated data de-identification techniques to protect patient identities while preserving scientific value. For global studies, we manage secure cross-border transfers using legally compliant mechanisms, ensuring data remains protected everywhere.
How do these platforms integrate with existing clinical systems?
Modern clinical trial solution platforms are designed to integrate with your current technology stack, not force a complete overhaul. This is achieved through an API-first architecture, which allows seamless connection with your existing EDC (Electronic Data Capture), RTSM/IRT (Randomization and Trial Supply Management), eTMF (electronic Trial Master File), and LIMS (Laboratory Information Management System) solutions.
A key capability is EHR/EMR integration for patient identification and eligibility matching. This can dramatically speed up recruitment by identifying potential participants directly from electronic health records while maintaining strict privacy protections. The flexibility of this approach means you can create a truly connected ecosystem, whether you replace all systems or integrate new capabilities gradually.
What is the implementation and change management process like?
A successful implementation of a new clinical trial solution follows a structured, supportive process. The journey begins with pre-protocol advisory services, where experts collaborate with your team to design optimal digital strategies before the protocol is finalized. This is followed by platform configuration to your study’s specific requirements and rigorous validation and User Acceptance Testing (UAT).
Effective change management is critical. We provide comprehensive, role-specific training and enablement to ensure all users are confident with the new workflows. Our support doesn’t end at go-live; ongoing support from dedicated implementation teams ensures a smooth transition. Our adoption strategies focus on demonstrating value quickly. When sites and sponsors see the immediate benefits, enthusiasm builds naturally, making the change feel empowering rather than disruptive.
Conclusion: Embracing the Next Generation of Clinical Research
The shift to a unified clinical trial solution is a fundamental reimagining of clinical research. It directly tackles today’s most pressing challenges: the patient burden that limits participation to under 3% and the administrative inefficiencies costing sponsors over $1 billion annually.
The benefits are clear. Patients can complete up to 74% of assessments from home, reporting over 60% improved experiences. Sites are freed from administrative chaos, and sponsors accelerate timelines by 6 months while saving over $25 million per trial in key therapeutic areas. These are not just metrics; they represent real progress in making research more efficient and human-centered.
The power of AI and federated data access is open uping previously impossible insights. By harmonizing clinical trial data with real-world evidence, we advance our understanding of how treatments work.
Looking ahead, the future is promising. AI copilots will assist researchers in making smarter decisions, while expanded integrations and digital endpoints will continue to push the boundaries of clinical development. The vision of personalized medicine is becoming a reality.
At Lifebit, we are actively building this future. Our federated AI platform delivers the secure data access, advanced analytics, and regulatory compliance that modern trials demand. By embracing these next-generation platforms, we can accelerate drug findy, improve patient outcomes, and make research more accessible to all.
The choice is to continue with fragmented systems or to accept a unified future that puts patients first. It’s time to modernize clinical development and lead the way to a healthier future.
Explore our unified platform for life sciences to see how we are building the future of trials.

