What Happens at a Clinical Trials Centre?

Clinical Trials Centre: 4 Essential Phases
Understanding the Hub of Medical Innovation
A clinical trials centre is a specialized facility where researchers conduct studies on new treatments, medications, and medical devices before they are publicly available. These centres are the critical link between laboratory findings and real-world patient care, carefully evaluating new therapies to ensure they are both safe and effective. The core functions of a clinical trials centre are comprehensive and interconnected. They include Research Coordination, which involves the complex logistical management of everything from single-site studies to sprawling multicentre, national, and international trials that can span continents. Specialized Patient Care is paramount, providing participants with protocol-driven medical support, continuous monitoring for safety, and management of any adverse events throughout the trial. Robust Data Management systems are in place to collect, clean, analyze, and report trial results according to strict compliance standards like HIPAA and GDPR, ensuring the integrity of the evidence generated. Furthermore, centres are responsible for Regulatory Compliance, navigating the intricate web of ethical guidelines and government regulations from bodies like the FDA and Health Canada to secure approvals and maintain oversight. Finally, they serve as hubs for Education and Training, actively developing the next generation of investigators and research staff through structured mentorship and continuous professional development programs.
Clinical trials centres operate in various settings, from hospital-based facilities like London Health Sciences Centre (with 2,789 active projects in 2023/24) to independent research organizations. The Centre for Clinical Trial Support at Sunnybrook Health Sciences Centre coordinates everything from single-centre studies to large international trials with significant clinical impacts.
These facilities are essential for advancing healthcare. For example, all new cancer treatments must be proven safe and effective in clinical trials before approval by Health Canada or the FDA.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in genomics and biomedical data, I’ve built platforms for precision medicine and data-driven drug findy. This experience gives me deep insight into how a clinical trials centre operates and the technology driving modern clinical research.
Clinical trials centre terms at a glance:
What is a Clinical Trials Centre and What is its Mission?
A clinical trials centre is a dynamic ecosystem dedicated to improving healthcare. Whether it’s housed within a hospital, an independent facility, or a Contract Research Organization (CRO), its core mission is to conduct rigorous, ethical scientific studies that advance medical knowledge and improve patient care. These centres are where groundbreaking ideas are tested, refined, and safely brought to patients.

The importance of clinical trials in medicine is immense. They form the backbone of evidence-based healthcare, providing the data needed to confirm if new treatments are safe and effective. From drug findy to post-market monitoring, a clinical trials centre plays an indispensable role. Learn more about the significance of clinical trials in medicine.
The Core Purpose: Advancing Healthcare
The primary mission of every clinical trials centre is to advance healthcare. The Centre for Clinical Trial Support (CCTS) at Sunnybrook Health Sciences Centre exemplifies this by helping researchers design, coordinate, and execute trials. Their purpose is to facilitate high-quality clinical research that can change lives.
This support covers a wide range of studies, from single-centre trials at one location to large multicentre, national, and international studies. This broad scope ensures research can tackle diverse health challenges and have a global impact. For instance, the CCTS has coordinated major international trials that have significantly impacted clinical practice.
At Lifebit, our work supporting researchers with secure access to biomedical data highlights how robust data management is key to these goals. It’s about celebrating the role of data in clinical trials to accelerate findies.
Guiding Principles and Values
The operations of a reputable clinical trials centre are built on strong core values that ensure ethical conduct, scientific integrity, and patient safety.
Data integrity is paramount. All data collected must be accurate, complete, and trustworthy, as trial results inform medical practices and regulatory approvals worldwide. This is governed by principles like ALCOA-C, which dictates that data must be Attributable, Legible, Contemporaneous, Original, Accurate, and Complete. Processes like source data verification (SDV), where data entered into the trial database is checked against original source documents, are fundamental to upholding this standard. Hand-in-hand with this is compliant conduct, meaning strict adherence to all regulations and guidelines, such as Good Clinical Practice (GCP), which provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions.
Ethics are the moral bedrock of clinical research. Every trial must be approved by an independent research ethics board (REB) or institutional review board (IRB) to protect the rights, safety, and well-being of participants. This includes a meticulous informed consent process, which is not merely a form to be signed but an ongoing dialogue between the research team and the participant. It ensures participants fully understand the trial’s purpose, procedures, potential risks and benefits, alternative treatments, and their right to confidentiality and to withdraw at any time without penalty.
Finally, a key value is fostering learning, mentoring, and education for investigators. Centres like the CCTS actively invest in developing researchers, knowing that continuous learning and professional development are essential for keeping pace with evolving technologies, methodologies, and regulations, improving research quality and building the next generation of clinical trial leaders.
The Inner Workings of a Modern Clinical Trials Centre
Ever wondered what happens inside a clinical trials centre? It’s a hub of coordinated activity, where dedicated teams and precise processes work together to bring new medical treatments to life.
Here, we’ll explore the essential services these centres offer, the key personnel involved, and how they ensure everything is done safely and correctly to deliver trustworthy medical breakthroughs.
Key Services and Support for Researchers
A clinical trials centre provides comprehensive services to guide studies from concept to conclusion, ensuring they run smoothly and effectively.
- Trial Design: Experts help researchers plan the study, defining goals, methodology, and participant criteria. A solid plan is crucial for meaningful results.
- Regulatory Submission: The centre handles submissions to bodies like Health Canada, the FDA, and ethics boards, ensuring all legal and ethical requirements are met before a trial begins.
- Budgeting and Contract Negotiation: Developing detailed trial budgets and negotiating contracts with sponsors and vendors to ensure financial viability.
- Project Management: Project managers oversee timelines, budgets, and communication, keeping complex, often multi-site, projects on track.
- Participant Recruitment and Retention: Designing and implementing strategies to find, enroll, and retain eligible participants, one of the most challenging aspects of clinical research.
- Data Management and Analysis: This involves collecting, cleaning, and organizing trial data. Experts then analyze it to answer research questions. Our work at Lifebit, with our Clinical Research SaaS Technology, is vital here, helping researchers access and analyze data securely.
- Results Reporting: The centre assists with writing reports and publishing findings to share new knowledge with the medical community and the public.
These services support all trial types, from single-centre to multicentre, national, and international studies. For example, the Canadian Centre for Clinical Trials (CCCT) offers custom support for medical research, handling everything from budgets to trial management. This allows researchers to focus on the science.
The People Behind the Research: Key Roles and Personnel
Behind every successful trial is a skilled, collaborative team of professionals.
- Principal Investigators (PIs): The lead researchers (doctors or scientists) who design and oversee the entire study, ensuring ethical conduct and patient safety.
- Study Coordinators: They manage daily operations, including patient recruitment, scheduling, data collection, and ensuring protocol adherence.
- Research Nurses: They provide direct patient care, administer treatments, monitor for side effects, and collect important health data.
- Data Managers: These experts handle all trial information, ensuring it is accurate, complete, and secure.
- Biostatisticians: Experts in statistical methodology who are critical for trial design, determining sample size, and analyzing data to produce valid conclusions.
- Clinical Research Associates (CRAs): Also known as monitors, they travel to trial sites to verify that the study is being conducted according to the protocol, GCP, and regulatory requirements.
- Regulatory Specialists: They ensure the trial complies with all local, national, and international regulations.
- Leadership Teams: Directors and managers guide the centre’s overall strategy, operations, and resource management, like the team at Sunnybrook’s Centre for Clinical Trial Support (CCTS).
This team effort is vital for conducting trials to the highest standards. Learn more on innovations in clinical trial recruitment.
Ensuring Quality and Compliance in a clinical trials centre
Quality and compliance are non-negotiable for trial results to be trusted. A clinical trials centre operates in a highly regulated environment to protect participants and ensure scientific validity.
Good Clinical Practice (GCP) is an international ethical and scientific quality standard for trials. Adherence protects participants’ rights and safety and ensures data reliability.
Research Ethics Boards (REBs), or Institutional Review Boards (IRBs), are independent committees that review and approve trial plans before they begin and monitor ongoing studies to ensure patient safety and ethical conduct. For instance, London Health Sciences Centre (LHSC) notes that an independent REB oversees all its trials.
Official bodies like Health Canada and the U.S. Food and Drug Administration (FDA) set the legal framework for clinical trials, which centres must follow carefully.
Data security is also critical. Centres must use robust methods like secure storage, anonymization, and strict access controls to protect sensitive patient information. At Lifebit, we build secure data platforms for clinical trial success to handle medical data with the highest level of security.
Looking ahead, the new ICH E6(R3) GCP Guidelines will modernize this framework. A key evolution is the emphasis on a risk-based approach to trial management and quality. This encourages sponsors and centres to identify critical-to-quality factors and focus monitoring and resources on the risks that matter most to patient safety and data integrity. This shift away from one-size-fits-all procedures allows for more efficient, flexible, and technology-enabled trials while reinforcing the core principles of quality management and data integrity.
For Patients: Participating in a Clinical Trial
Participating in a clinical trial is a significant decision that offers personal benefits and the chance to contribute to medical advancements. A clinical trials centre provides a supportive environment to guide you through this journey.

This section explains the patient experience, outlining the benefits, what to expect, and how to find and enroll in trials. Our goal is to empower you with clear information to make confident healthcare choices.
Benefits and What to Expect
Joining a clinical trial offers unique advantages. As organizations like the London Health Sciences Centre (LHSCRI) highlight, key benefits include:
- Accessing cutting-edge treatments: You may receive new therapies not yet widely available, which is especially valuable for conditions with limited treatment options.
- Contributing to research: Your participation directly helps advance medical knowledge and develop more effective treatments for future patients.
- Receiving additional support: Participants often receive close monitoring and comprehensive care from a dedicated research team of doctors, nurses, and coordinators.
It’s also important to understand potential risks. While trials prioritize patient safety, new treatments can have side effects or may not be effective for everyone. The informed consent process is crucial; it ensures you are fully aware of all potential benefits and risks before deciding to participate. This discussion should involve you, your family, and your healthcare provider.
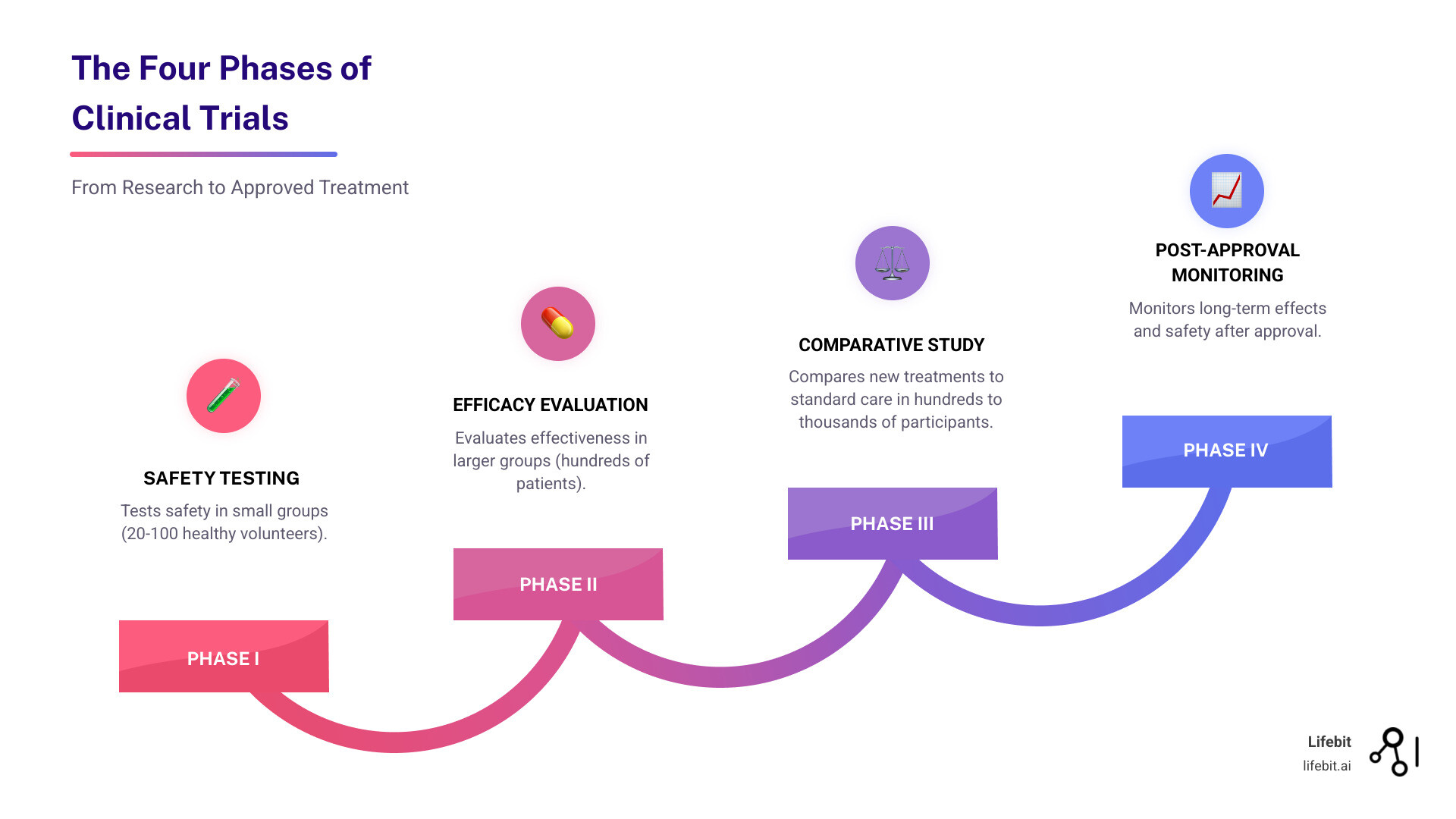
Your journey in a trial typically includes screening to confirm eligibility, baseline health assessments, receiving the study intervention, regular follow-up visits, and a final assessment. The research team is there to support you and answer questions throughout the process. Your experience will also depend on the trial’s phase. Phase I trials are the first step in human testing, involving a small group of participants to evaluate safety, determine a safe dosage range, and identify side effects. Phase II trials expand to a larger group to test effectiveness and further evaluate safety. Phase III trials are large-scale studies involving hundreds to thousands of participants to confirm effectiveness, monitor side effects, compare it to standard treatments, and collect information that will allow the new treatment to be used safely. If successful, the treatment may be approved for public use. Phase IV trials occur after the treatment is marketed to monitor its long-term effectiveness and safety in diverse populations. The benefits of real-world data in clinical research show how patient contributions are shaping the future of medicine.
How to Find a clinical trials centre and Enrol
Finding and enrolling in a trial starts with a proactive approach, usually beginning with a conversation with your doctor. Here are the key steps:
- Speak with Your Doctor: Your doctor knows your medical history and can advise if a trial is a suitable option, help you understand the details, and determine your eligibility. For example, at Princess Margaret Cancer Centre, doctors typically refer and enrol patients.
- Use Online Trial Registries: These databases list ongoing trials worldwide.
- ClinicalTrials.gov: A U.S. National Library of Medicine registry with over 40,000 trials. You can search for trials on ClinicalTrials.gov by condition or location.
- Clinical Trials Ontario (CTO): A platform to find trials in Ontario, which has over 1,000 active trials.
- Other Registries: Health Canada’s Clinical Trials Database, the WHO International Clinical Trials Registry Platform (ICTRP), the ISRCTN registry, and CenterWatch are also excellent resources.
- Contact Specific Research Centres: You can also contact specialized research centres or hospitals known for work in your condition. Many have dedicated units to answer questions about clinical research.
- Review and Discuss Trial Details: Once you find potential trials, review the details with your healthcare team. This is crucial for understanding the commitment, risks, and if the trial aligns with your treatment plan and goals.
Key Questions to Ask
Before enrolling, be well-informed. Consider asking the research team these questions:
- What is the main purpose of this study?
- What tests, procedures, and treatments are involved? How do they differ from my current care?
- What are the potential short-term and long-term risks and benefits for me?
- How much of my time will be required? Will I have to travel or be hospitalized?
- Will I have to pay for any part of the trial? Will I be reimbursed for expenses like travel?
- What happens if I am harmed during the study?
- How will my personal information be kept confidential?
- What happens after the trial ends? Will I continue to receive the treatment?
The decision to participate is personal and should always be made in consultation with your healthcare team, ensuring you feel fully informed.
Global Collaboration and Future Trends
Clinical research is a global, collaborative effort. No single clinical trials centre works in isolation; they are part of a vast network of teamwork and shared knowledge, all aiming to improve global health.
This section explores how these centres contribute to the global research community and what the future holds for clinical research, driven by new technology and a patient-centric focus. At Lifebit, we are proud to support these emerging trends & technologies in clinical trial design.
Fostering a Global Research Ecosystem
Collaboration is the soul of modern clinical research. Centres team up with partners worldwide because tackling major health challenges requires diverse expertise and patient populations.
Organizations like the International Clinical Trial Center Network (ICN) connect centres and promote high standards. In Canada, Clinical Trials Ontario (CTO) has been vital in growing the province’s trial landscape by streamlining ethics reviews, accelerating trial start-up, guiding researchers, mentoring talent, and engaging the public. This has helped make Ontario a leader in Canadian clinical research, home to four of the country’s top 40 research hospitals. However, this global collaboration is not without its challenges. Centres must steer a complex patchwork of different national regulatory requirements (e.g., FDA in the U.S. vs. the European Medicines Agency, EMA), varying ethical review standards, and stringent data privacy laws like HIPAA and GDPR. Successfully managing these problems is a testament to the sophisticated capabilities of modern clinical trial networks.
Sunnybrook Health Sciences Centre’s Medical Oncology & Hematology division is a prime example. This clinical trials centre is a research hub with over 300 publications and $20 million in funding. Its team members hold leadership roles in influential groups like Cancer Care Ontario’s Program in Evidence-Based Care (CCO-PEBC) and the National Cancer Institute of Canada-Clinical Trials Group (NCIC-CTG), influencing cancer care practices nationally and globally.
Their research excellence spans areas like Cancer Immunotherapy, Chronobiology (exploring optimal timing for cancer treatments in collaboration with groups like the EORTC), Translational Research, and Quality Improvement Initiatives. This deep involvement shows how individual centres contribute to a thriving global research family and highlights the need to break barriers and increase diversity in clinical trials.
The Future: Technology-Driven and Patient-Centric Trials
The future of clinical research is more efficient, accessible, and patient-focused, thanks to new technologies and a renewed emphasis on the patient experience. A clinical trials centre will continue to evolve by embracing these innovations.
Decentralized trials (DCTs) use a suite of technologies like eConsent, telehealth appointments, remote monitoring sensors, and direct-to-patient shipment of study drugs to reduce or eliminate the need for clinic visits. This not only makes participation more convenient and accessible for a broader range of patients but also helps improve the diversity of trial populations. Our decentralized clinical trials guidance explores this transformative shift.
Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing nearly every stage of the trial process. For example, AI algorithms can scan millions of electronic health records in minutes to identify eligible patients for a trial—a task that would take humans months. ML models can predict patient dropout rates, optimize trial designs in real-time (adaptive trials), and improve pharmacovigilance by detecting safety signals from vast datasets far earlier than traditional methods.
Wearable technology provides continuous, real-time data on participants’ health (e.g., activity, sleep, heart rate). This offers a richer understanding of a treatment’s effects compared to occasional clinic visits.
Real-World Data (RWD), from sources like electronic health records, provides valuable insights into how treatments perform in everyday clinical practice, complementing traditional trial data. Enabling secure access to RWD is a core part of our mission at Lifebit.
Finally, there is a major push for greater patient diversity and inclusion. Future trials will actively recruit participants from diverse backgrounds to ensure new treatments are effective for everyone. This patient-first approach, combined with technology, will make clinical trials more impactful than ever.
Conclusion
A clinical trials centre is a hub of hope and innovation, bridging the gap between scientific findy and patient care. These centres are the engines of medical progress, essential for rigorously testing new treatments to ensure they are both safe and effective before they reach the public.
Operating through robust global partnerships and collaborations, these centres—from large academic hospital-based units to specialized independent facilities—are unified in their mission to improve patient outcomes. Organizations like Sunnybrook’s Centre for Clinical Trial Support (CCTS), the Canadian Centre for Clinical Trials (CCCT), and Clinical Trials Ontario (CTO) exemplify this collaborative spirit.
At their heart, these centres are driven by unwavering principles of data integrity, strict ethical standards grounded in patient protection, and a culture of continuous learning. For patients, understanding the trial phases and their rights allows them to engage with a clinical trials centre as empowered partners, gaining access to cutting-edge treatments while making an invaluable contribution to medical science.
Looking ahead, the future is bright. The adoption of risk-based quality management, alongside transformative innovations like decentralized trials, AI-driven analytics, wearable technology, and Real-World Data, is revolutionizing how research is conducted. These advancements will empower every clinical trials centre to fulfill its critical mission with greater efficiency, inclusivity, and impact.
At Lifebit, we are proud to empower this ecosystem with our next-generation federated AI platform. It enables secure, real-time access to global biomedical data for large-scale, compliant research and safety surveillance. With advanced AI/ML analytics and federated governance, we help transform data into life-changing insights. Our platform components, including the Trusted Research Environment (TRE), Trusted Data Lakehouse (TDL), and R.E.A.L. (Real-time Evidence & Analytics Layer), deliver real-time insights and secure collaboration.
A clinical trials centre represents the best of human ingenuity and compassion. Through these tireless efforts, we continue to build a healthier, more hopeful future for everyone. Find how federated technology is changing research and empowering this essential work.


