From Complex to Clear: A Guide to Clinical Trial Efficiency

Why Clinical Trial Efficiency Matters Now More Than Ever
Clinical trial efficiency is the ability to deliver safe, effective treatments to patients faster and at lower cost. Here’s what that means in practice:
- Cut activation time from 784 days to under 450 days
- Reduce trial costs from $31.7 million median per oncology trial
- Accelerate patient recruitment using AI and predictive analytics
- Streamline data management to eliminate 15+ portal logins per study
- Leverage real-world evidence to improve generalizability and speed approvals
Clinical trials are the backbone of modern medicine. But they’re also slow, expensive, and logistically complex. A single Phase 3 trial can take over 370 distinct processes and more than two years just to activate. Protocol amendments have increased by 60% in seven years, while implementation time has nearly tripled. Sites juggle over 15 portals per study, with password changes every six to eight weeks, leading to repeated data entry and undermined quality.
These inefficiencies don’t just waste resources—they delay life-saving treatments. In oncology alone, the median cost per pivotal trial reached $31.7 million between 2015 and 2017. Meanwhile, 21% of patients in early-phase trials remain in active follow-up for months after treatment ends, creating unnecessary administrative burden.
The good news? New approaches are changing the game. Adaptive trial designs, AI-powered patient matching, decentralized models, and real-world evidence are cutting timelines and costs while maintaining scientific rigor. Innovations like basket and umbrella trials allow targeted therapies to be tested across multiple tumor types or molecular subsets simultaneously. Predictive analytics can now identify eligible patients in seconds instead of months. And connected data systems are eliminating the fragmented tools that slow everything down.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over a decade building federated platforms that accelerate clinical trial efficiency through secure, AI-powered data analysis. Our work with global pharma, public sector institutions, and regulatory bodies has shown how the right infrastructure can transform time-to-insight from months to minutes.

Simple guide to Clinical trial efficiency terms:
Identifying the Bottlenecks Killing Your Clinical Trial Efficiency
To fix a problem, we first have to see it clearly. For many of us in the industry, the “old way” of running trials feels like being stuck in a bad escape room. We are surrounded by data, yet we can’t find the key to move to the next phase.
Research shows that the complexity of clinical trials is increasing across all indications. This isn’t just a feeling; it’s a measurable trend. We are seeing more endpoints, more eligibility criteria, and more data points collected per patient than ever before. While this is driven by a desire for scientific rigor, it often results in a “technology burden” that actually slows us down.
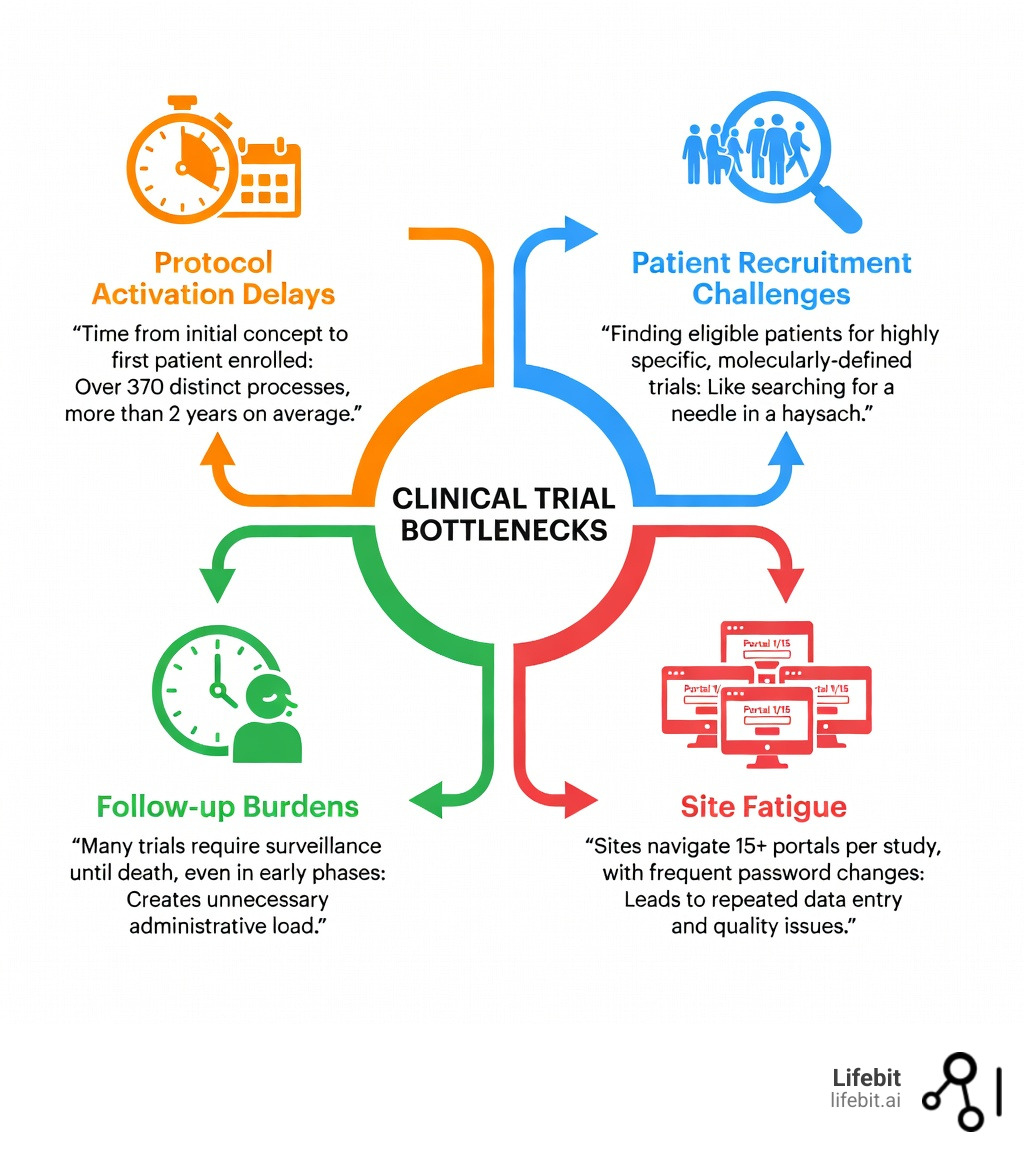
The primary bottlenecks we face today include:
- Protocol Activation Delays: The time from initial concept to the first patient enrolled is staggering.
- Patient Recruitment Challenges: Finding the right patients for highly specific, molecularly-defined trials is like finding a needle in a haystack.
- Follow-up Burdens: Many trials mandate prolonged surveillance until death, even in early phases where it might not be scientifically necessary.
- Site Fatigue: Staff are forced to steer dozens of portals, each with its own login and unique data standards.
According to scientific research on Phase 3 trial activation timelines, the median duration to activate a trial was 784 days. That is over two years of administrative work before the first patient even receives a dose.
Solving the 784-Day Activation Delay
We believe that the concept-to-activation timeline is the biggest area for improvement. The delay is rarely caused by the science itself; it’s usually administrative. Parallel processing is the key here. Instead of waiting for one department to finish a review before starting the next, we advocate for multidisciplinary collaboration where legal, financial, and ethical reviews happen simultaneously.
Initiatives like the NCI’s Operational Efficiency Working Group (OEWG) have set aggressive targets—400 to 450 days for Phase 1 and 2 trials—to force this change. By adopting a clinical trial solution that centralizes these workflows, we can cut through the red tape.
Reducing Protocol Complexity and Amendments
Every time a protocol is amended, the clock restarts. Over the last seven years, the number of amendments per protocol has jumped by 60%. Worse, the time it takes to implement these changes has nearly tripled. This doesn’t just cost money; it undermines data quality.
| Feature | Traditional Management | Streamlined Management |
|---|---|---|
| Protocol Design | Rigid, fixed from start | Adaptive, data-driven |
| Site Interaction | 15+ portals, manual entry | Unified data systems |
| Amendments | Slow, manual rollout | Rapid, digital implementation |
| Data Quality | High risk of manual error | Automated cleaning & validation |
By focusing on essential data points and using clinical trial data management tools that allow for flexible updates, we can reduce the site burden and keep the trial on track.
Implementing Innovative Designs to Accelerate Precision Medicine
The era of “one-size-fits-all” medicine is over. Today, we are targeting specific genetic mutations, which means our trial designs must be as precise as the drugs we are testing. Traditional trial models are often too slow to handle the molecular heterogeneity of modern diseases, especially in clinical cancer research.
Innovative designs like basket trials, umbrella trials, and adaptive enrichment are the new gold standard.
- Basket Trials: These allow us to test a single targeted therapy across multiple tumor types that share the same molecular feature.
- Umbrella Trials: These focus on one tumor type but test multiple different drugs based on the specific molecular subsets of the patients.
- Adaptive Enrichment: These designs use interim data to narrow down the patient population to those most likely to benefit, saving time and resources.
Lessons from NCI MATCH and Lung MAP
Major initiatives like NCI’s MATCH and Lung MAP have proven that these master protocols work. By using a centralized infrastructure for molecular screening, these trials can assign patients to the right sub-study in a fraction of the time it would take to screen them for individual trials. This collaborative approach is essential for clinical trial efficiency in precision medicine.
Using Biomarkers to Personalize Clinical Trial Efficiency
Biomarkers are the compass of modern trials. They tell us who to treat and how they are responding. However, the pace of biomarker findy is often too slow. We use AI-powered biomarker discovery to integrate multi-modal data—genomic, imaging, and clinical—to identify predictive signatures faster. This personalization ensures that we aren’t wasting time on patients who won’t respond, significantly improving the trial’s scientific efficiency.
Leveraging AI and Predictive Analytics for Faster Patient Matching
If protocol activation is the engine of a trial, patient recruitment is the fuel. Without participants, the trial stalls. Currently, patient recruitment accounts for nearly 30% of trial timelines. AI and predictive analytics are changing this by moving us away from manual chart reviews toward automated matching.
Predictive analytics allows us to:
- Forecast recruitment rates based on historical data.
- Identify “exceptional responders” in real-world datasets.
- Proactively predict adverse events to improve patient safety and retention.
Optimizing Recruitment with Machine Learning
We use machine learning to scan electronic health records (EHRs) and identify eligible participants in seconds. This is particularly vital for improving diversity. By analyzing data across different geographic regions and demographics, we can ensure that trial populations are truly representative. For a deep dive, see our AI clinical trial recruitment ultimate guide.
Enhancing Clinical Trial Efficiency Through Automated Screening
According to scientific research on AI boost for clinical trials, automated screening tools can dramatically increase the number of eligible patients identified. Instead of relying on a busy doctor to remember a trial, AI for clinical trials acts as a 24/7 matching engine, flagging patients the moment their data hits the system.
Streamlining Operations with Decentralized Models and Real-World Evidence
The COVID-19 pandemic taught us that trials don’t always have to happen in a hospital. Decentralized clinical trials (DCTs) use digital health tools—like wearables and telemedicine—to bring the trial to the patient. This reduces the “participation burden,” making it easier for people to stay in the study.
Integrating Real-World Data for Better Generalizability
Traditional trials happen in “ideal” conditions, but real life is messy. By integrating Real-World Data (RWD) from EHRs, insurance claims, and patient registries, we can see how a drug performs in a broader population. This improves the generalizability of our findings and can even be used to create “synthetic control arms,” reducing the number of patients who need to be recruited for a placebo group. Learn more about real-world data in clinical trials to see how this accelerates regulatory approvals.
Reducing Site Burden with Connected Data Systems
We have all heard of “password fatigue.” Site staff often steer 15+ portals per study. This isn’t just annoying; it leads to data entry errors. Our approach at Lifebit is to use federated, connected data systems. Instead of moving data into different silos, we bring the analysis to the data. This eliminates repeated data entry and ensures that everyone is working from a single, harmonized “truth.”
For more on managing these complex workflows, check out our decentralized clinical trials guidance.
Frequently Asked Questions about Clinical Trial Efficiency
What are the primary causes of inefficiency in clinical trials?
The main culprits are administrative delays during protocol activation, overly complex eligibility criteria that slow down recruitment, and the use of fragmented legacy “site portals” that increase manual workload for staff.
How do adaptive trial designs improve drug development timelines?
Adaptive designs allow researchers to make pre-planned changes to the trial based on interim data. This might include stopping a treatment arm that isn’t working or increasing the sample size for a promising one, effectively combining Phase 2 and Phase 3 and cutting months or years off the timeline.
What role does real-world evidence play in modern trial efficiency?
Real-world evidence (RWE) helps by providing data on how drugs work in everyday clinical settings. It can be used to support regulatory submissions, monitor long-term safety without requiring new trials, and even replace traditional control groups in certain rare disease or oncology studies.
Conclusion
The future of clinical trial efficiency lies in breaking down the walls between data silos and embracing a more flexible, AI-driven approach. We are seeing a shift toward global collaboration and virtual models that prioritize the patient experience while maintaining the highest scientific standards.
At Lifebit, we are proud to be part of this change. By providing a federated AI platform that enables secure, real-time access to global data, we are helping our partners turn complex science into clear results. The road to faster drug findy is paved with better data, smarter designs, and a commitment to removing the bottlenecks that stand in the way of patient care.
Ready to optimize your next study? Read our white paper on Designing Clinical Trials for Optimal Success.

