The Vigilant Eye: How Post-Marketing Surveillance Keeps Drugs Safe

Post marketing drug surveillance: Critical for Safer Drugs 2025
Why Drug Safety Surveillance Never Stops
Post marketing drug surveillance is the systematic monitoring of approved drugs to detect, assess, and prevent adverse effects once they reach real-world patients. Here’s what you need to know:
Key Components:
- Passive surveillance – Voluntary reporting of adverse events by healthcare providers and patients
- Active surveillance – Proactive data collection through registries, cohort studies, and electronic health records
- Signal detection – Using statistical methods and AI to identify potential safety concerns
- Regulatory action – Label updates, safety warnings, or drug withdrawals when needed
Primary Goals:
- Detect rare adverse effects not seen in clinical trials
- Monitor long-term safety in diverse populations
- Identify drug interactions and contraindications
- Ensure benefits continue to outweigh risks
The stakes couldn’t be higher. As one regulatory expert noted: “All possible side effects of a drug can’t be anticipated based on preapproval studies involving only several hundred to several thousand patients.” This is why continuous monitoring becomes essential once drugs enter the broader market.
Clinical trials involve carefully selected participants under controlled conditions. But real-world use reveals how drugs perform across diverse populations, age groups, and medical conditions – often uncovering safety signals that weren’t apparent during development.
The numbers tell the story: In 2017 alone, Health Canada received 860,000 adverse reaction reports and reviewed 166 safety issues, resulting in 44 safety reviews and 137 risk communications to healthcare providers.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built federated data platforms that power real-time pharmacovigilance across secure healthcare environments. My experience in computational biology and biomedical data integration has shown me how post marketing drug surveillance transforms from a regulatory requirement into a lifesaving early warning system when powered by the right technology.
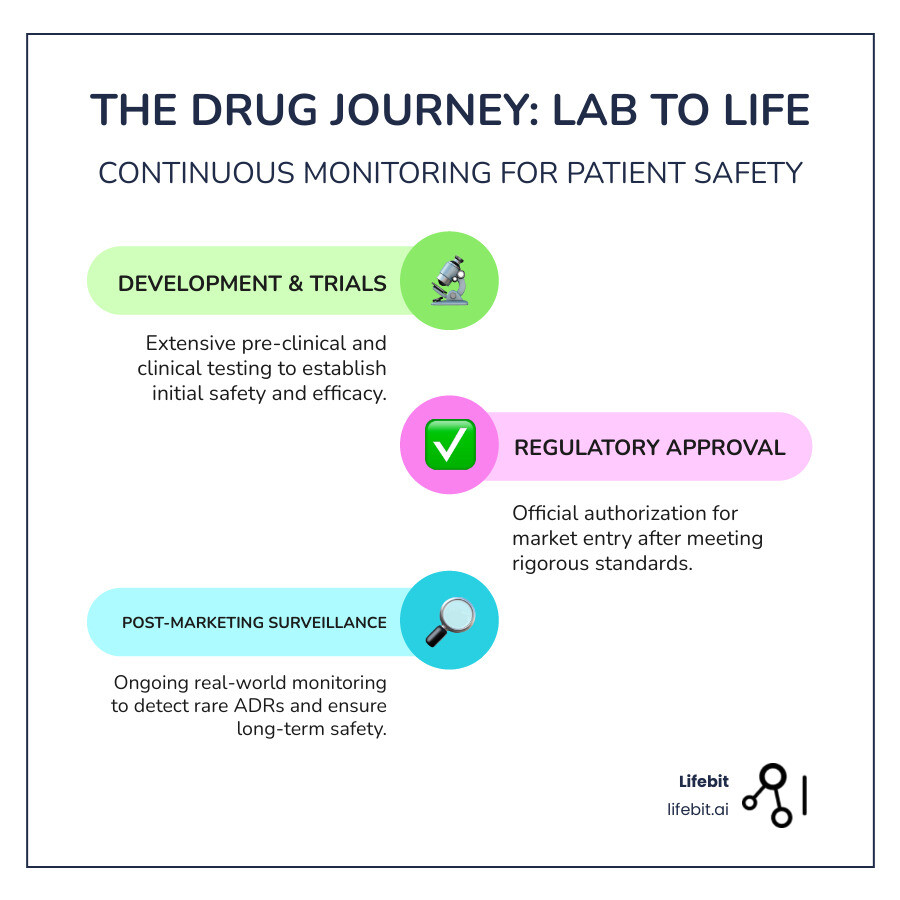
Why Drug Safety Doesn’t End at Approval
Getting a drug approved is like graduating from medical school – it’s a huge milestone, but it’s really just the beginning of the journey. When regulatory agencies give their stamp of approval, they’re saying “this drug looks safe and effective based on what we know so far.” But here’s the thing: post marketing drug surveillance is where the real learning begins.
Think of it this way – clinical trials are like test-driving a car on a closed course with professional drivers. Everything’s controlled, predictable, and carefully monitored. But once that car hits real roads with everyday drivers in all kinds of weather and traffic conditions? That’s when you find what really happens.
What is Post-Marketing Surveillance?
Post marketing drug surveillance is essentially keeping a watchful eye on medications after they’ve left the safety of clinical trials and entered the real world. It’s part of the broader field of pharmacovigilance – the science of monitoring drug safety throughout a medication’s entire lifecycle. The need for this field was tragically highlighted in the 1960s with the thalidomide disaster. Thalidomide was marketed as a safe sedative for pregnant women but was later found to cause severe birth defects. This event became a powerful catalyst, leading to the establishment of modern drug safety regulations and the formal systems for post-marketing surveillance we rely on today.
When we talk about the drug development process, most people know about Phase I, II, and III trials. Post marketing drug surveillance is often called Phase IV, and it’s arguably the most important phase of all. Why? Because this is when millions of real patients – not carefully selected trial participants – start using the medication in their daily lives.
Clinical trials might involve a few thousand people over a couple of years. But once a drug hits the market, it could be used by millions of people over decades. These real-world patients have different genetic backgrounds, take other medications, have various health conditions, and live completely different lifestyles than trial participants.
This ongoing monitoring helps us spot rare side effects that might only show up in 1 out of 10,000 patients. You’d never catch those in a trial of 3,000 people. It also reveals long-term effects that take years to develop, unexpected drug interactions, and how the medication performs when doctors prescribe it for conditions beyond its original approval.
At Lifebit, we’ve seen how Real-time Pharmacovigilance can transform this process, making it faster and more accurate than ever before.
The Importance of Continuous Monitoring
Here’s why we can’t just approve a drug and call it a day. Clinical trials, despite being incredibly rigorous, have some pretty significant limitations when it comes to predicting real-world performance.
Limited scope is the biggest issue. Trials typically exclude patients who are pregnant, elderly, have multiple health conditions (comorbidities), or take several other medications (polypharmacy). This is done to isolate the drug’s effect and protect vulnerable individuals, but it means the trial population is not representative of the real world. But guess what? Those are exactly the people who’ll be using the drug once it’s approved.
Time constraints create another blind spot. Most trials last months or maybe a few years. But some side effects, particularly those related to chronic diseases like cancer or cardiovascular issues, only show up after a decade of use. Others might be linked to specific genetic variations that weren’t well-represented in the original study population.
Then there’s off-label use – when doctors prescribe medications for conditions they weren’t originally approved to treat. This happens all the time in medicine, and it’s often beneficial. But we need post marketing drug surveillance to make sure these uses are safe too.
The Vioxx case perfectly illustrates why this monitoring matters. This popular painkiller (a COX-2 inhibitor) sailed through clinical trials and was used by millions. However, post-marketing data, including a long-term clinical trial called APPROVe, eventually revealed a significantly increased risk of heart attacks and strokes with long-term use. The drug was pulled from the market in 2004, a move that likely prevented tens of thousands of adverse cardiovascular events. Another prominent example is rosiglitazone (Avandia), a diabetes drug. Post-marketing analysis of clinical trial data suggested an increased risk of heart attack, leading the FDA to place severe restrictions on its use for many years, demonstrating a regulatory action short of complete withdrawal.
This is where the Benefits of Real-World Data in Clinical Research become crystal clear. We get the complete picture of how drugs actually perform in the messy, complex world of everyday healthcare.
Goals and Objectives
Post marketing drug surveillance has several key objectives, all focused on keeping patients safe while ensuring they can access effective treatments.
Detecting rare adverse effects is probably the most critical goal. If a serious side effect happens in 1 out of 50,000 patients, you’ll never see it in a trial of 5,000 people. But when millions start using the drug, these rare events become visible and actionable.
Identifying new risk factors helps us understand which patients might be more vulnerable to side effects. Maybe the drug is perfectly safe for most people but risky for those with a specific genetic variation (pharmacogenomics) or a particular comorbidity like kidney disease.
Confirming real-world effectiveness ensures the drug actually works as expected outside the controlled environment of clinical trials. Sometimes drugs perform differently when used by diverse populations with varying health conditions.
Monitoring long-term safety catches problems that only emerge after years of use. Some effects are cumulative, building up slowly over time until they become clinically significant.
Updating drug labels keeps prescribing information current and comprehensive. As we learn more about a drug’s real-world performance, we update warnings, precautions, and usage guidelines. In some cases, this may involve adding a Boxed Warning (also known as a “black box warning”), the FDA’s most serious type of warning, to highlight life-threatening risks. For example, many antidepressants carry a boxed warning about increased risk of suicidal thoughts in children and young adults.
Informing regulatory decisions provides the evidence regulators need to take action when necessary. This might mean issuing safety warnings, restricting how a drug can be used, or in extreme cases, removing it from the market entirely.
The ultimate goal is maintaining the right benefit-risk balance. Every medication has some level of risk – even aspirin can cause serious bleeding in some people. Post marketing drug surveillance helps ensure that for each drug on the market, the benefits clearly outweigh the risks for the patients who use it.
The Core Methods of Post-Marketing Drug Surveillance
Think of post marketing drug surveillance as a detective story that never ends. Once a drug hits the market, we need multiple ways to gather clues about how it’s really performing in the wild. The methods we use range from waiting for people to report problems voluntarily, to actively hunting down information like digital bloodhounds.
Passive Surveillance: The Foundation of Reporting
Imagine if you only heard about problems when people took the time to call you. That’s essentially how passive surveillance works – and it’s been the backbone of post marketing drug surveillance for decades.
Spontaneous Reporting Systems (SRSs) are the workhorses here. Healthcare professionals, patients, and pharmaceutical companies can voluntarily submit reports when they suspect a drug caused an adverse reaction. It’s like having millions of watchful eyes around the world, all connected to central databases.
The FDA MedWatch program in the United States lets anyone report serious reactions, product quality problems, or therapeutic failures. These reports flow into FAERS (the FDA Adverse Event Reporting System), a massive computerized database that supports safety surveillance for all approved drugs and biologics. Across the pond, the UK Yellow Card Scheme has been collecting reports since the 1960s. It was one of the first systems to let both healthcare professionals and patients report suspected side effects. Meanwhile, Health Canada operates its own vigilance system, creating a global network of safety monitoring.
However, SRSs have a major catch: underreporting. Studies suggest that only 1-10% of all adverse events are ever reported. Healthcare providers might be too busy, unsure if the drug was the cause, or assume someone else reported it. There’s also reporting bias, where severe or unusual events are reported more often than common, mild ones, skewing the data.
Signal Detection and Data Mining
Because of the sheer volume and noise in these databases, simply counting reports isn’t enough. This is where signal detection comes in. A signal is defined as reported information on a possible causal relationship between an adverse event and a drug, which is unknown or incompletely documented previously. To find these signals, regulators and manufacturers use sophisticated data mining algorithms to perform disproportionality analysis. These methods compare the frequency of a specific drug-event combination in the database to the frequency one would expect by chance. Common techniques include calculating a Reporting Odds Ratio (ROR) or a Proportional Reporting Ratio (PRR). A value above a certain threshold acts as a statistical flag, suggesting a potential association that warrants further investigation. It’s crucial to remember that a statistical signal is not proof of causation; it is an early warning that requires rigorous clinical and epidemiological evaluation.
Despite its limitations, passive surveillance remains essential. It’s cost-effective, covers the entire population, and has successfully identified serious safety signals that led to life-saving regulatory actions. For a comprehensive look at how this works in practice, check out this research on Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting.
Active Surveillance: Proactively Seeking Data
If passive surveillance is waiting for the phone to ring, active surveillance is making the calls. It involves proactively collecting data to get a more complete and accurate picture.
Observational studies, such as cohort and case-control studies, are a cornerstone of active surveillance. Cohort studies are particularly powerful; they can be prospective, where researchers identify a group of users (a cohort) and follow them forward in time to see what outcomes develop, or retrospective, using existing data like health records to compare outcomes between those who did and did not take a drug.
Patient registries are specialized databases for specific drugs, diseases, or populations (e.g., pregnant women). For example, the North American AED (Antiepileptic Drug) Pregnancy Registry prospectively tracks outcomes for pregnant women taking seizure medications, providing crucial safety data that could never be obtained from a clinical trial.
Prescription event monitoring involves systematically following up with doctors or patients after a new drug is prescribed to collect data on all adverse events, not just those suspected to be drug-related.
The real game-changer has been Electronic Health Records (EHRs) and claims data. The FDA Sentinel Initiative is a prime example. It’s a distributed data network that can query the anonymized records of over 300 million patients from major health systems and insurers. The key is that the data remains securely behind each partner’s firewall; Sentinel sends queries to the data, and only receives back aggregate results, protecting patient privacy while enabling rapid safety analysis.
This is where modern technology really shines. At Lifebit, we’ve seen how Optimizing Real-World Evidence in Pharma through these active methods can transform safety monitoring from reactive to proactive.
Challenges and Limitations in Post-Marketing Drug Surveillance
Even with these sophisticated methods, post marketing drug surveillance faces stubborn challenges.
Data complexity and integration tops the list. We’re dealing with information from spontaneous reports, EHRs, claims, registries, and even social media. Integrating these disparate sources is a massive technical hurdle.
Underreporting and data quality remain persistent headaches. Spontaneous reports can be incomplete, and EHR data can be messy or use inconsistent coding, leading to missed signals or false alarms.
Causality assessment is perhaps the trickiest challenge. Just because an event occurred after taking a drug doesn’t mean the drug caused it (post hoc ergo propter hoc fallacy). To move from correlation to potential causation, epidemiologists often use frameworks like the Bradford Hill criteria, which include factors like the strength of association, consistency across studies, temporality (cause before effect), and biological plausibility.
The Role of Social Media and Unstructured Data: A new frontier and challenge is the analysis of patient-generated data from social media (Twitter, Reddit) and online health forums (PatientsLikeMe). This unstructured data offers a wealth of potential insights but is incredibly noisy. Advanced Natural Language Processing (NLP) and machine learning techniques are being developed to extract potential adverse event signals from this text, but verifying the information and distinguishing it from casual conversation remains a significant challenge.
Resource intensity makes comprehensive surveillance difficult. Active surveillance methods, in particular, require substantial investments in technology, infrastructure, and specialized expertise.
The Global Regulatory Framework and Key Stakeholders
When it comes to post marketing drug surveillance, we’re dealing with a truly global effort. Think of it as a worldwide safety net, with regulatory agencies, pharmaceutical companies, and healthcare providers all working together to keep medications safe for everyone who needs them.

How Regulatory Bodies Manage Surveillance
Regulatory agencies are the watchful guardians of drug safety. The FDA in the United States, the EMA in Europe, and Health Canada each have their own approaches, but they all share the same goal: protecting public health through rigorous surveillance.
The FDA operates some of the world’s most comprehensive surveillance systems. Beyond MedWatch and FAERS, the FDA can require manufacturers to implement Risk Evaluation and Mitigation Strategies (REMS) for drugs with serious safety concerns. A REMS goes beyond standard labeling to ensure a drug’s benefits outweigh its risks. For example, the iPLEDGE REMS for the acne drug isotretinoin requires strict pregnancy testing and contraception counseling to prevent severe birth defects.
Across the Atlantic, the EMA coordinates surveillance across all European Union member states through its EudraVigilance database. This centralized system allows for powerful signal detection across a diverse population of nearly 450 million people.
Health Canada requires manufacturers to submit Risk Management Plans (RMPs) that outline how a product’s risks will be monitored and minimized. This emphasizes a lifecycle approach to drug safety, ensuring continuous evaluation of the benefit-risk balance.
International collaboration is key. The International Council for Harmonisation (ICH) brings together regulatory authorities and the pharmaceutical industry to create harmonized guidelines. For example, the ICH E2B guideline standardizes the format for transmitting Individual Case Safety Reports (ICSRs), allowing safety data to be shared seamlessly across borders. You can learn more about these coordinated efforts through Postmarketing Surveillance Programs – FDA.
Manufacturer and Hospital Responsibilities
While regulatory agencies set the rules, pharmaceutical companies and healthcare providers are on the front lines.
Pharmaceutical manufacturers have extensive obligations. They must collect and report adverse events from all sources globally. They are also required to submit Periodic Benefit-Risk Evaluation Reports (PBRERs), formerly known as Periodic Safety Update Reports (PSURs). These are comprehensive documents that analyze the cumulative safety data for a drug and provide an updated assessment of its benefit-risk profile. Companies invest heavily in signal detection, using statistical methods and increasingly, artificial intelligence and machine learning, to proactively scan databases for potential safety concerns before they become widespread problems.
Healthcare providers and hospitals are the foundation of the system. Their clinical judgment and willingness to report suspected adverse reactions provide the raw data that fuels all surveillance activities. The responsibility doesn’t end with reporting. Healthcare systems must also stay informed about safety updates, implement new precautions (like those mandated by a REMS), and educate their staff about emerging safety concerns. This creates a continuous feedback loop that strengthens the entire surveillance system. Health Canada provides excellent guidance on this collaborative approach through their Health products post-market surveillance cycle – Canada.ca.
The Importance of Post-Marketing Drug Surveillance in Vulnerable Populations
Here’s where post marketing drug surveillance becomes even more critical. Vulnerable populations like children, the elderly, and pregnant women are often underrepresented in clinical trials, making post-marketing data our primary source of safety information.
Pediatric patients are not small adults; their bodies metabolize drugs differently. In the U.S., the Pediatric Research Equity Act (PREA) and the Best Pharmaceuticals for Children Act (BPCA) require or incentivize manufacturers to conduct pediatric studies, but much of the prescribing for children remains “off-label.” Post-marketing surveillance is essential for monitoring the safety of this off-label use and identifying adverse events unique to children.
Elderly patients often suffer from multiple chronic conditions and engage in polypharmacy (the simultaneous use of multiple drugs). This dramatically increases the risk of drug-drug interactions and adverse events. Post-marketing surveillance helps identify these risks. Tools like the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults are developed and updated using evidence gathered from real-world surveillance data to guide safer prescribing in this population.
Pregnant women are almost always excluded from pre-market clinical trials for ethical reasons. Therefore, nearly all information on the safety of medications used during pregnancy comes from post-marketing surveillance. Pregnancy exposure registries are a key tool; these are prospective observational studies that monitor the outcomes of women who take a specific drug during pregnancy. This data is vital for informing both patients and providers about the potential risks and benefits of treatment during pregnancy.
At Lifebit, we understand that effective surveillance of vulnerable populations requires sophisticated data integration and analysis capabilities. Our federated platform enables secure, real-time monitoring across diverse patient populations while maintaining the privacy protections these sensitive groups require. For more information about pediatric health considerations, visit More on pediatric health.

