The Open Secret: How to Access and Share Clinical Trial Data Effectively

Clinical Trial Data Access: Stop Waiting Months—Get Data Faster, Stay Compliant
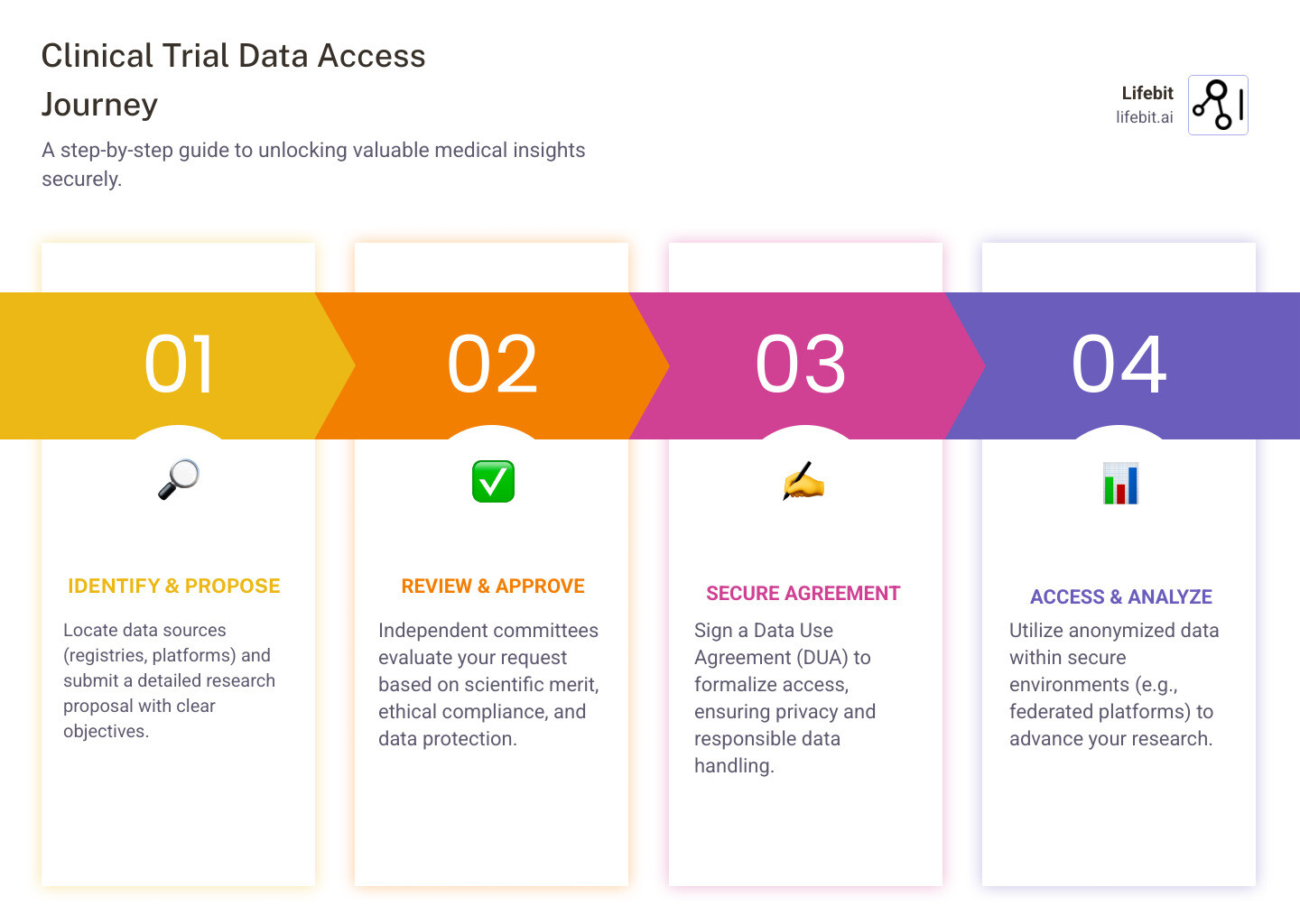
Clinical trial data access is the process of requesting, reviewing, and using anonymized patient-level information from completed or ongoing studies to advance research, validate findings, and improve patient care. Here’s how it works:
Quick Answer: How to Access Clinical Trial Data
- Choose a platform – Use registries like ClinicalTrials.gov or other established global data repositories
- Submit a request – Provide a research proposal with clear scientific objectives
- Sign a Data Use Agreement (DUA) – Commit to privacy protections and responsible use
- Get independent review – Your request is evaluated by data access committees
- Download or analyze data – Access datasets in secure environments or trusted research workspaces
Over 300,000 clinical studies are registered on ClinicalTrials.gov alone. Leading global platforms host data from thousands of trials involving millions of participants. Yet many researchers still wait weeks or months for approval – or never get access at all.
The stakes are high. When clinical trial data stays locked away, medical breakthroughs slow down. Researchers can’t validate findings. Patients miss out on safer, more effective treatments. And healthcare systems waste resources on ineffective interventions.
But when data flows freely – under the right protections – everything changes. Secondary analyses reveal hidden safety signals. Meta-analyses uncover treatment patterns across populations. And AI models trained on diverse datasets accelerate drug findy.
The challenge isn’t just getting access. It’s getting access fast, securely, and compliantly – without moving sensitive data or violating privacy regulations like HIPAA and GDPR.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built a federated platform that powers clinical trial data access for over 275 million patient records across 36+ countries. My team and I have spent 15 years solving the exact problems you’re facing: slow onboarding, poor data quality, and regulatory bottlenecks that delay findies.
Common clinical trial data access vocab:
Clinical Trial Data Access: Stop Letting Locked Data Delay Breakthroughs
In drug development, data is the most valuable currency we have. But if that currency is locked in a vault, it can’t generate interest. Clinical trial data access isn’t just a bureaucratic hurdle; it is the engine of modern medicine. Historically, the pharmaceutical industry operated in silos, with data often archived and forgotten once a primary study was completed. This “data hoarding” led to significant inefficiencies, where researchers would unknowingly duplicate efforts or miss critical safety signals that only become apparent when looking across multiple datasets.
When we talk about maximizing the value of a trial, we’re looking at secondary analysis. This is where researchers use existing data to answer new questions the original trial didn’t even ask. For example, a post-hoc analysis might reveal that a drug works better for a specific ethnic subgroup or identifies a previously unnoticed safety signal. According to research on responsible data sharing, sharing trial data maximizes the public’s investment and honors the contribution of the trial participants. A famous example is the SPRINT trial (Systolic Blood Pressure Intervention Trial), where the release of the data allowed dozens of independent teams to validate the findings and explore the impact of intensive blood pressure control on different age groups and comorbidities.
Beyond individual studies, access enables meta-analysis. By combining data from dozens of trials, we can see patterns that a single study—no matter how large—would miss. This is critical for validating treatment efficacy and ensuring patient safety. In fact, a lack of transparency has historically led to “invisible” trials where negative results were buried, skewing the medical literature and putting patients at risk. The “AllTrials” campaign has highlighted that roughly half of all clinical trials have never published their results, creating a massive gap in our medical knowledge. By opening access to this “dark data,” we can prevent the repetition of failed experiments and protect patients from ineffective treatments.
From a business perspective, the Research ROI is undeniable. Why spend $100 million on a new trial if the answer is already hidden in the patient-level data of three previous ones? By leveraging modern clinical research technology, we can turn “dead” data into living insights, accelerating the path from lab to life. Furthermore, the 21st Century Cures Act in the US and the EMA Policy 0070 in Europe have created regulatory mandates that make data sharing not just a moral choice, but a legal expectation for modern drug developers.
Clinical Trial Data Access: Choose Open vs. Controlled to Cut Risk
Not all data access is created equal. Depending on the sensitivity of the trial and the goals of the sponsor, access usually falls into one of two buckets: Open Access or Controlled Access. Understanding the nuances between these models is essential for both data providers and data consumers to ensure that scientific progress does not come at the cost of participant privacy.
Open Access vs. Controlled Access
| Feature | Open Access | Controlled Access |
|---|---|---|
| Who can access? | Anyone with an internet connection | Qualified researchers with approved proposals |
| Privacy Risk | Higher (harder to prevent re-identification) | Lower (mitigated by agreements and review) |
| Speed | Instant | Takes weeks/months for review |
| Examples | Summary results on ClinicalTrials.gov | Major industry-led data repositories |
| Data Depth | Aggregate/Summary level | Individual Patient Level Data (IPD) |
| Governance | Minimal | High (Independent Review Panels) |
While Open Access sounds ideal for transparency, it’s rarely used for individual patient-level data (IPD) due to privacy concerns. Most industry leaders prefer Controlled Access. This model uses Independent Review Panels (IRPs) or Data Access Committees (DACs) to vet every request. These panels check if the research question is scientifically valid and if the researchers have a sound statistical plan.
To manage this effectively, many organizations are adopting the “Five Safes” framework, which is a world-leading standard for managing data access:
- Safe People: Are the researchers qualified and trustworthy?
- Safe Projects: Is the research proposal scientifically sound and in the public interest?
- Safe Settings: Is the data being analyzed in a secure environment (like a TRE)?
- Safe Data: Has the data been appropriately de-identified?
- Safe Outputs: Are the results checked to ensure no individual can be identified before they are published?
To make this work, we use secure data environments that allow researchers to analyze the data without ever downloading it. This is a “data-to-code” approach rather than “code-to-data,” ensuring the sponsor maintains governance oversight while the researcher gets the insights they need. This model also prevents the “leakage” of data, as the raw datasets never leave the controlled infrastructure of the data custodian.
Clinical Trial Data Access: Prevent Re-Identification Before It Derails Your Study
The biggest “boogeyman” in clinical trial data access is the risk of re-identification. Even when names and social security numbers are removed, a clever attacker could combine trial data with other public records (like voter registration or social media) to identify a participant. Research on re-identification risks shows that while actual attacks are rare, the theoretical risk is real, especially in the era of “Big Data.” This is often referred to as the “Mosaic Effect,” where multiple pieces of non-identifiable information are combined to create a identifiable profile.
To fight this, we employ several layers of defense that go beyond simple anonymization:
- De-identification and Anonymization: This involves stripping direct identifiers and “blurring” indirect ones. For example, instead of a specific birthdate, we use a birth year. Instead of a specific zip code, we use a broader region. We also use techniques like k-anonymity, which ensures that any individual in a dataset cannot be distinguished from at least k-1 other individuals.
- Differential Privacy: This is a mathematical approach that adds a specific amount of “noise” to the data. This noise is enough to protect individual identities but not enough to skew the overall statistical results of the analysis. It is currently considered the gold standard for privacy-preserving data sharing.
- Data Use Agreements (DUAs): These are legally binding contracts where researchers promise not to attempt re-identification and to report any safety signals they find. These agreements often include heavy penalties for misuse, providing a strong legal deterrent against unethical behavior.
- Federated Governance: This is our specialty at Lifebit. Instead of moving data to a central server, we keep the data where it lives (e.g., at the hospital or the pharma company). The analysis happens locally, and only the results are shared. This significantly lowers the risk of a massive data breach because there is no central “honeypot” of data for hackers to target.
- Airlocks and Output Checking: These act as digital security guards. When a researcher finishes their analysis, the results must pass through an “airlock” where they are reviewed—either manually or automatically—to ensure they only contain aggregate statistics and no patient-level information.
By combining these with HIPAA analytics best practices and GDPR-compliant workflows, we can build a system of trust where patients feel safe contributing their data to science. This multi-layered approach ensures that the utility of the data is preserved while the privacy of the individual remains paramount.
Clinical Trial Data Access: How to Request Data (and Avoid Rejection)
If you’re a researcher looking to dive into these datasets, you need a plan. You can’t just send an email saying, “I’d like some data, please.” The process is rigorous for a reason, and understanding the expectations of the review panels can significantly increase your chances of approval.
The Standard Request Workflow
- Identify Your Source: Start with registries like the WHO International Clinical Trials Registry Platform (ICTRP) or the EU Clinical Trials Register. For industry-specific data, platforms like Vivli, ClinicalStudyDataRequest.com (CSDR), and the YODA Project (Yale University Open Data Access) are the primary gateways to datasets from companies like GSK, Johnson & Johnson, and Pfizer.
- Draft a Robust Research Proposal: This is the most important document. It must include:
- Scientific Objective: What specific question are you trying to answer?
- Statistical Analysis Plan (SAP): You must detail the exact methods you will use. Vague plans like “exploratory analysis” are often rejected.
- Publication Plan: How and where do you intend to share your findings?
- Research Team Credentials: Who will be handling the data, and what is their experience?
- Submit for Review: Your proposal goes to an independent review panel. They will evaluate it for scientific merit, potential conflicts of interest, and whether the data requested is actually necessary to answer the question. They also check if the proposed research overlaps with ongoing studies to avoid redundant work.
- Execute the DUA: Once approved, you and your institution sign the Data Use Agreement. This step can sometimes take time as legal departments negotiate terms, so it’s best to involve your institution’s legal counsel early in the process.
- Access the Secure Workspace: You’ll likely be given a login to a clinical trial data management platform. Here, you’ll find the raw data, the Data Dictionary (which explains what each variable means), and the Case Report Forms (CRFs) used during the trial. You will also have access to tools like R, Python, or SAS to run your analysis.
Common Reasons for Rejection
- Lack of Scientific Rigor: The proposal doesn’t have a clear hypothesis or the math doesn’t add up.
- Privacy Concerns: The request asks for data that is too granular, increasing re-identification risk.
- Conflict of Interest: The researcher has a financial stake that could bias the results.
- Data Not Available: Sometimes, data from older trials or trials with very small participant numbers cannot be shared due to consent limitations.
At Lifebit, we support this by providing Trusted Research Environments (TREs) that make this entire process seamless. Instead of wrestling with different file formats or slow download speeds, researchers can access harmonized data in a workspace that’s already compliant with global regulations, allowing them to focus on the science rather than the logistics.
Clinical Trial Data Access: Why Shipping Data Is Dead—and What Replaces It
The old way of sharing data—shipping hard drives or massive FTP transfers—is dead. It is slow, insecure, and creates multiple “versions of the truth” that are impossible to track. The future of clinical trial data access is Federated Learning and decentralized analysis.
Imagine a world where a researcher in London can run an AI model across trial data stored in New York, Singapore, and Canada simultaneously, without any of that data ever leaving its home country. This is the power of a trusted research environment. It allows for global collaboration on a scale we’ve never seen before. This is particularly vital for rare disease research, where no single hospital or country has enough patients to conduct a statistically significant study. By federating access, we can create a “virtual cohort” of thousands of patients across the globe.
We are also seeing a massive shift toward real-world data (RWD). Clinical trials are controlled environments, but RWD (from EHRs, insurance claims, and wearable devices) shows how a drug works in the “wild.” The next frontier is integrating these RWD sources with clinical trial IPD and multi-omic data (genomics, proteomics, metabolomics). This creates a “Digital Twin” of the patient—a comprehensive digital model that can be used to simulate how a specific individual might respond to a treatment before it is even prescribed.
Furthermore, the European Health Data Space (EHDS) is a major regulatory initiative that aims to create a single market for health data across the EU. This will standardize how clinical trial data is accessed and shared across borders, removing the current patchwork of national regulations that often stymie international research.
Finally, patient advocacy groups are taking a seat at the table. Patients no longer want to be “subjects”; they want to be partners. This means more transparency in how data is used and more “plain English” summaries of trial results. We are seeing the rise of “Participant-Led Research,” where patients themselves propose the questions they want researchers to answer using their data. This shift ensures that clinical research remains focused on the outcomes that matter most to the people living with the conditions being studied.
Clinical Trial Data Access: Answers on Speed, Ethics, and Review Panels
What are the main goals of clinical trial data access?
The primary goals are to accelerate scientific advancement, improve patient care, and foster transparency. By allowing secondary use of data, we can confirm the original study’s results (reproducibility) and find new insights that lead to better treatments. It also builds public trust by showing that the risks participants took were not in vain. Furthermore, it promotes the FAIR principles—making data Findable, Accessible, Interoperable, and Reusable—which is the cornerstone of modern open science.
How do independent review panels oversee the process?
Independent review panels (IRPs) act as the “gatekeepers.” They are typically composed of experts in medicine, statistics, and ethics, often including lay representatives or patient advocates. They review requests for scientific merit (is this a real question?), ethical compliance (does this protect patients?), and statistical rigor (is the math right?). Their goal is to prevent the data from being used for “fishing expeditions”—where researchers keep testing variables until they find a random correlation—or other forms of invalid analysis that could lead to false medical claims.
What are the ethical considerations in clinical trial data access?
The core ethical challenge is balancing the social benefit of research with the autonomy and privacy of the participant. This involves ensuring that the original informed consent covers secondary use and that the data is handled in a way that prevents harm (like stigmatization or insurance discrimination). There is also the “moral imperative” to share data—it is arguably unethical to keep data hidden if it could save lives. Another emerging ethical issue is data equity: ensuring that researchers from low-and-middle-income countries have the same access to these global datasets as those in wealthy institutions.
Who owns the intellectual property (IP) in shared data?
Generally, the original sponsor (e.g., the pharma company) retains ownership of the raw data. However, the researcher who performs the secondary analysis typically owns the new insights or algorithms they develop from that data, provided they don’t violate the terms of the Data Use Agreement. Most DUAs require that any new findings be published openly to benefit the wider scientific community, rather than being kept as trade secrets.
How long does it typically take to get access?
In a traditional model, it can take anywhere from 3 to 9 months to move from proposal to data access. This includes time for the review panel to meet, legal teams to sign off on the DUA, and IT teams to provision the data. However, using federated platforms like Lifebit, this timeline can be reduced to weeks, as the legal and technical frameworks are often pre-negotiated and standardized.
Clinical Trial Data Access: Stop Losing Insights—Build a Learning Health System
The ultimate goal of clinical trial data access is to create a “learning system.” This is a virtuous cycle where every trial informs the next, every patient’s experience contributes to a global knowledge base, and every researcher has the tools they need to succeed.
At Lifebit, we believe that the “Open Secret” to better medicine is collaboration. By using federated trusted research environments, we can break down the silos that have held back research for decades. We are moving from a world of “my data” to a world of “our insights.”
Are you ready to stop waiting for data and start making breakthroughs? The technology is here. The data is waiting. Lets get to work.
Ready to scale your research with secure, federated clinical data? Explore the Lifebit Platform or Contact Us to see a demo of our Trusted Research Environments in action.


