Real-Time Adverse Drug Reaction Surveillance Platform
Automated ADR Signal Detection for Real-Time Pharmacovigilance
Lifebit’s platform sets a new standard in real-time adverse drug reaction surveillance, empowering regulatory and research bodies with immediate insights.

Lifebit R.E.A.L.
Real-Time Adverse Drug Reaction Surveillance Platform. Lifebit’s platform sets a new standard in real-time adverse drug reaction surveillance, empowering regulatory and research bodies with immediate insights.

- 🧠 AI-Driven, Real-Time ADR Surveillance
Smarter pharmacovigilance for public health and safety
Lifebit R.E.A.L. turns global real-world data into instant insights—automatically detecting adverse drug reactions (ADRs), surfacing trends, and prioritizing risks without delays.
-
Eliminate manual review
-
Reduce costs
-
Prioritize security
-
Safeguard patients
- Why Lifebit R.E.A.L.?
Your trusted partner for real-time pharmacovigilance
Powering trusted ADR insights for the world’s leading public health agencies and pharma innovators.
AI-powered signal detection at scale
Detect emerging adverse drug reactions (ADRs) in real time using AI-driven analysis of global structured and unstructured data—minimizing manual review delays.
Built for regulatory compliance and speed
Fully compliant with FedRAMP, HIPAA, GDPR, and FDA guidelines, Lifebit R.E.A.L. delivers secure, real-time surveillance across health and research ecosystems.
Trusted by governments and global leaders
Selected by NIH, Singapore’s Ministry of Health, and Boehringer Ingelheim, Lifebit R.E.A.L. powers scalable ADR monitoring and safety insights worldwide.
Impact
Accelerate signal detection and optimize post-market safety monitoring with Lifebit R.E.A.L.™
Lower Surveillance Costs
Up to 30%
savings with federated compute, cloud resource optimization, and intelligent cost governance.
AI-Powered Detection
< 24 hours
to detect new adverse events using federated AI across structured and unstructured data sources.
Compliant Reproducibility
100%
traceability across case clustering, model-based signal evaluation, and exportable reports with full audit trails.
How it Works.
We’ve outlined how Lifebit R.E.A.L.™ helps organizations unlock real-time adverse event insights from complex, distributed data—securely and at scale.
Log in and define your safety focus.
Start by accessing the Lifebit R.E.A.L.™ platform. Simply specify the drug(s), therapeutic class, population, or event type you wish to monitor. The system instantly configures real-time surveillance parameters—no code or setup needed.
Track emerging risks, instantly.
The platform continuously scans global data—clinical notes, public health records, and spontaneous reporting systems—to surface real-time adverse drug reactions (ADRs) across populations and geographies.
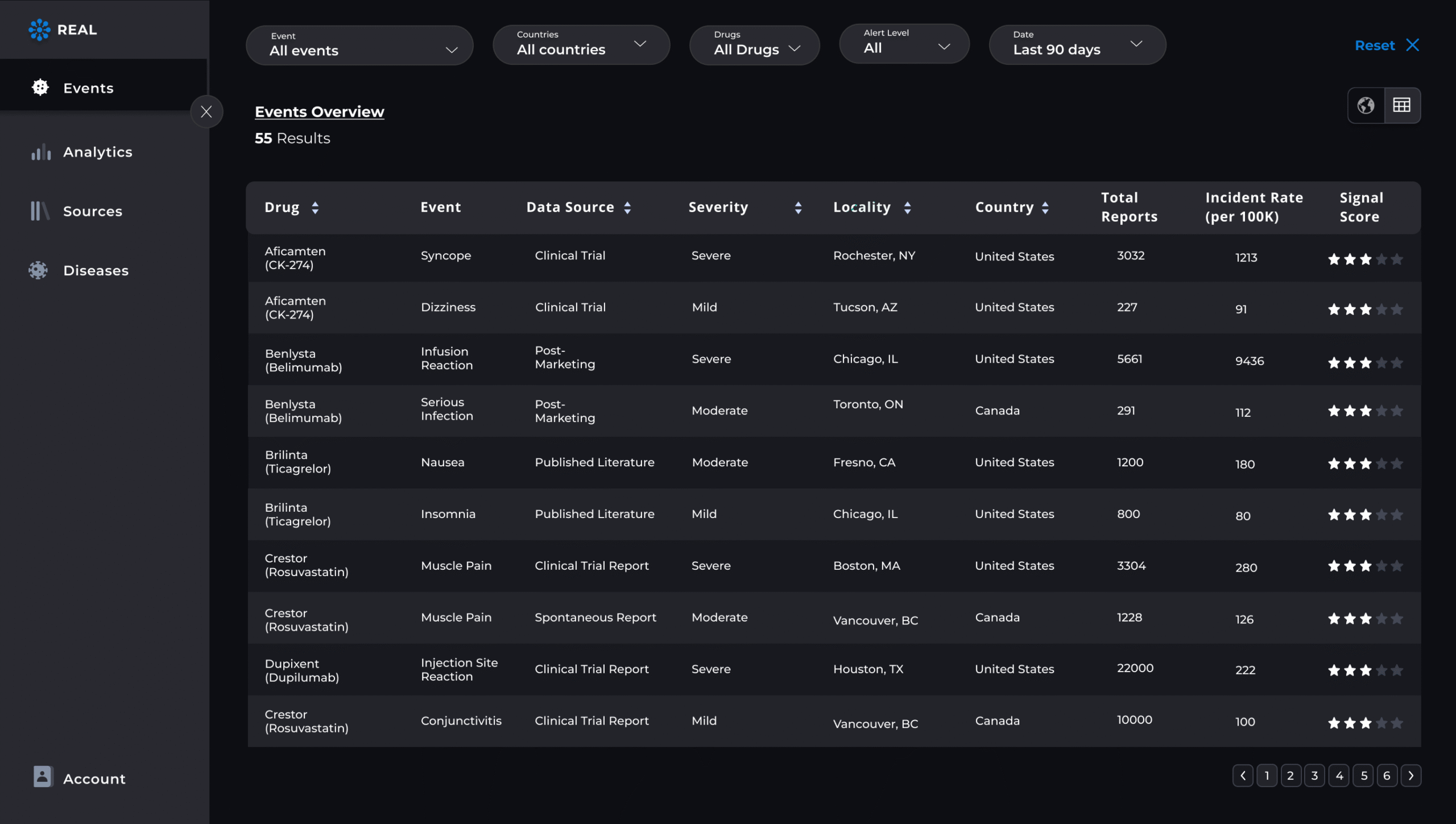
Detect ADR signals in real-time
Lifebit continuously monitors global data sources—from EHRs and safety databases to literature and social media—for emerging adverse drug reactions (ADRs), keeping you ahead of potential threats.
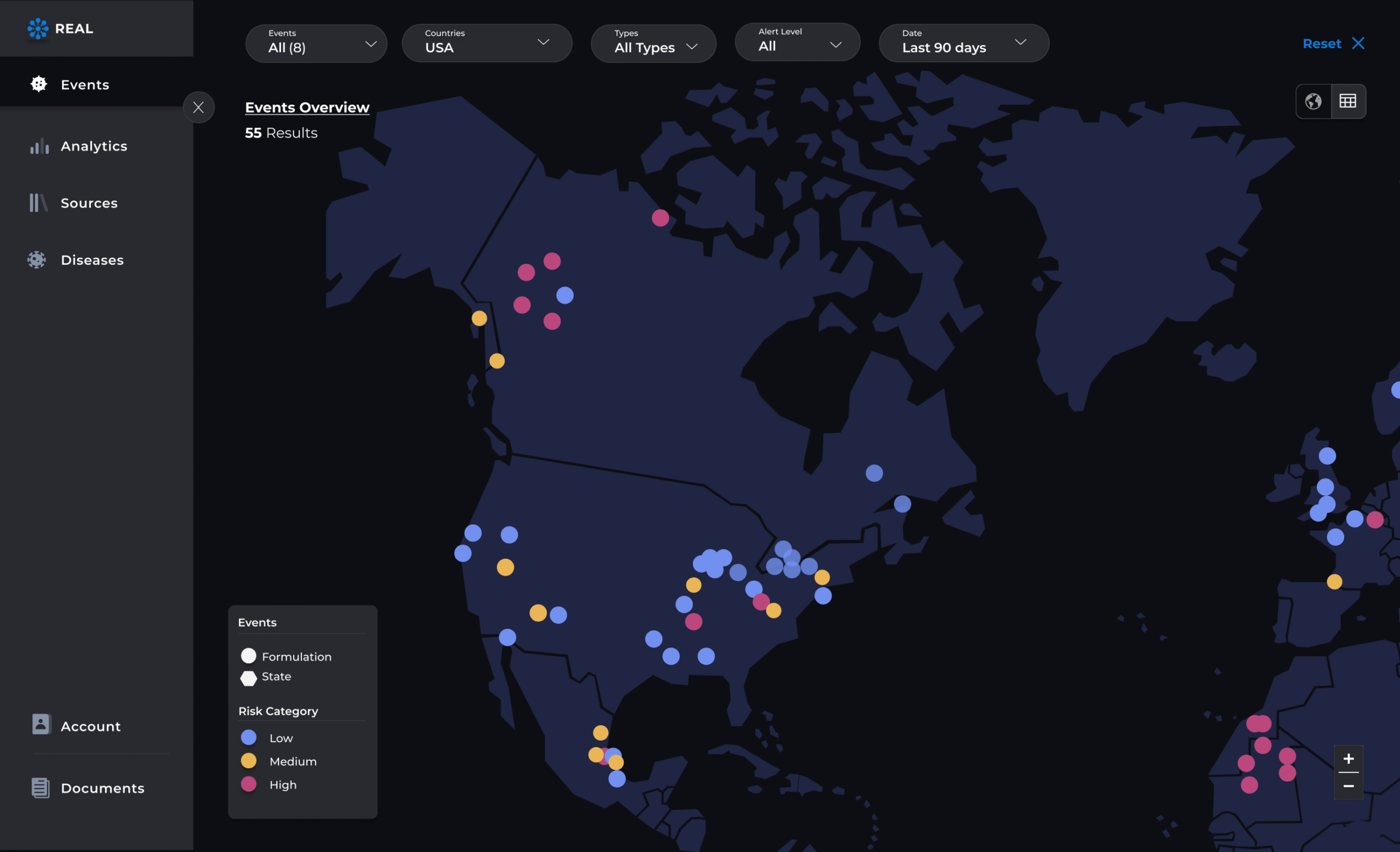
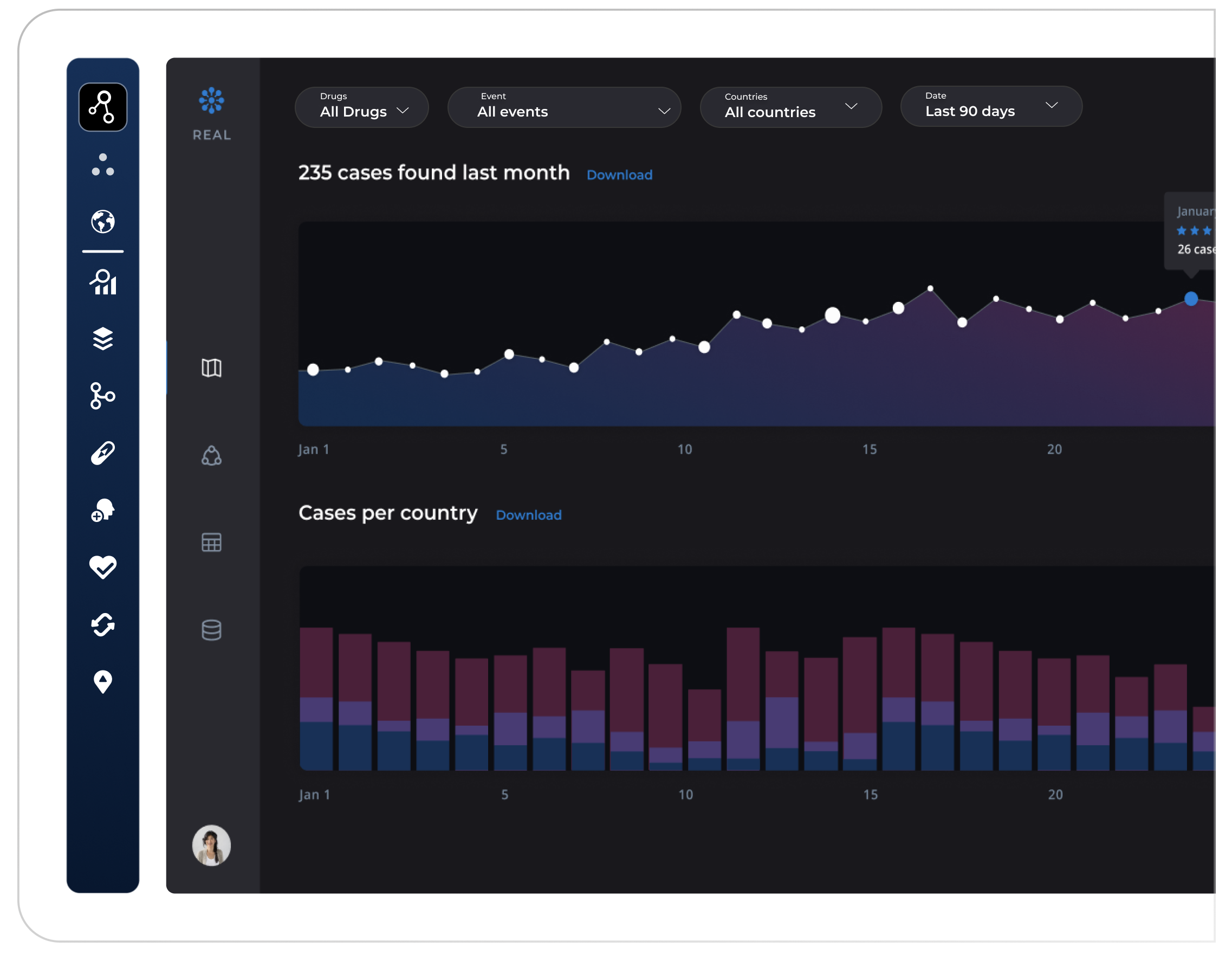
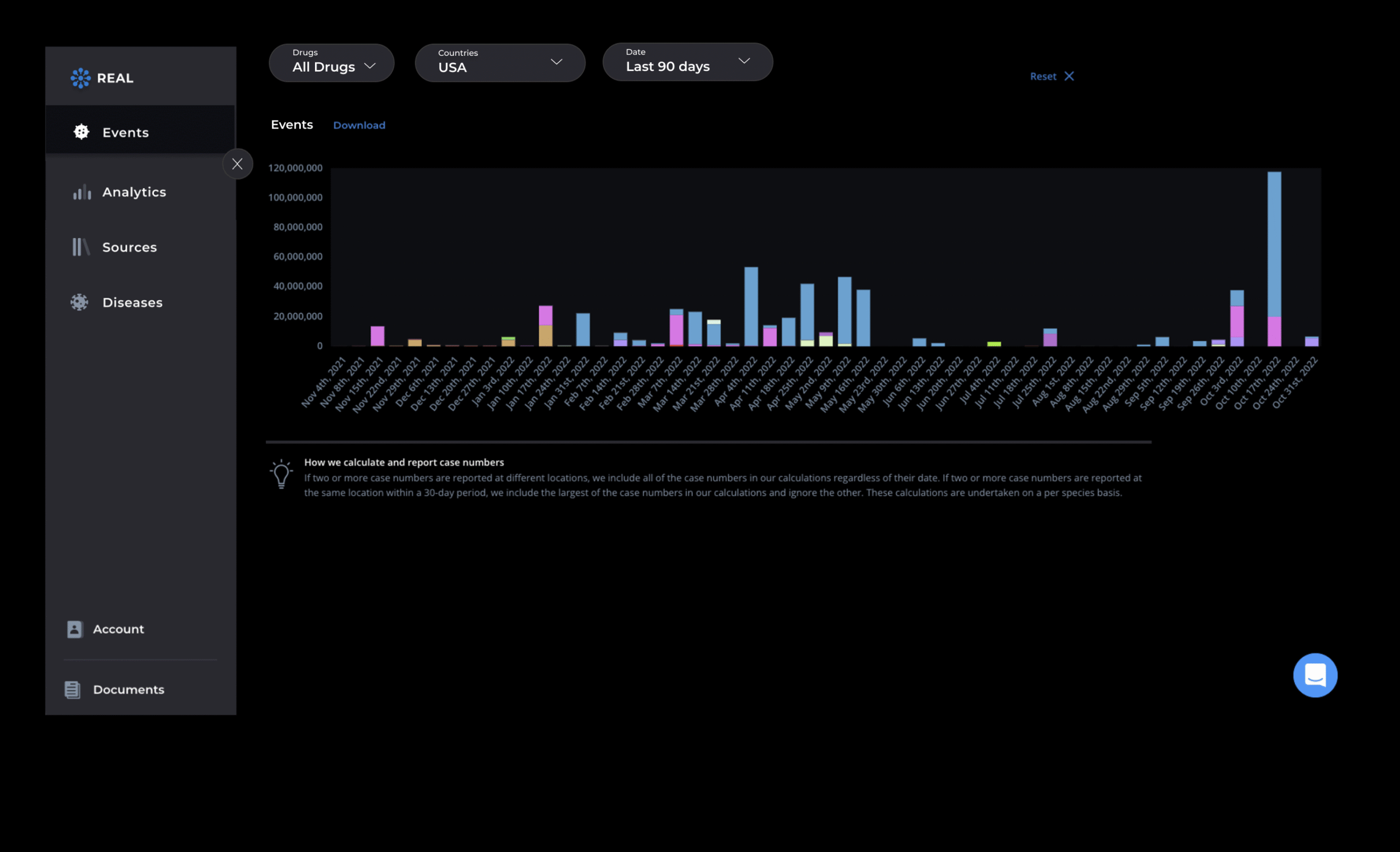
Visualize activity and signal clusters
Interactive dashboards display geographic case maps, spike alerts, and granular case-level data with intuitive filters by drug, demographic, region, or clinical outcome—enabling instant signal tracking, risk assessment, and proactive action planning.
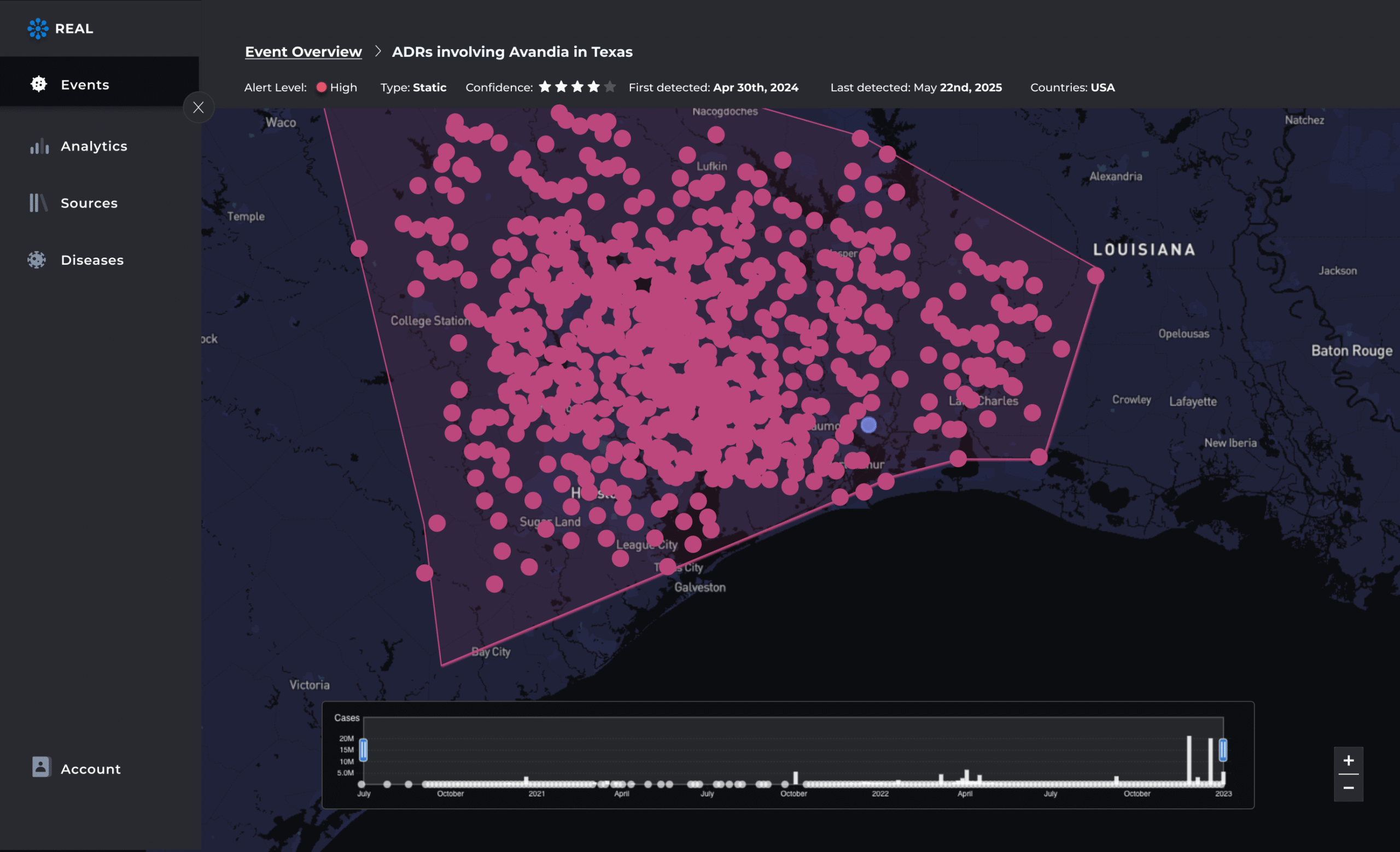
Prioritize and triage emerging ADRs
AI models rank signals by severity, frequency, and novelty—so you know what matters most. Drill into timelines, supporting evidence, and affected populations to support faster decisions.
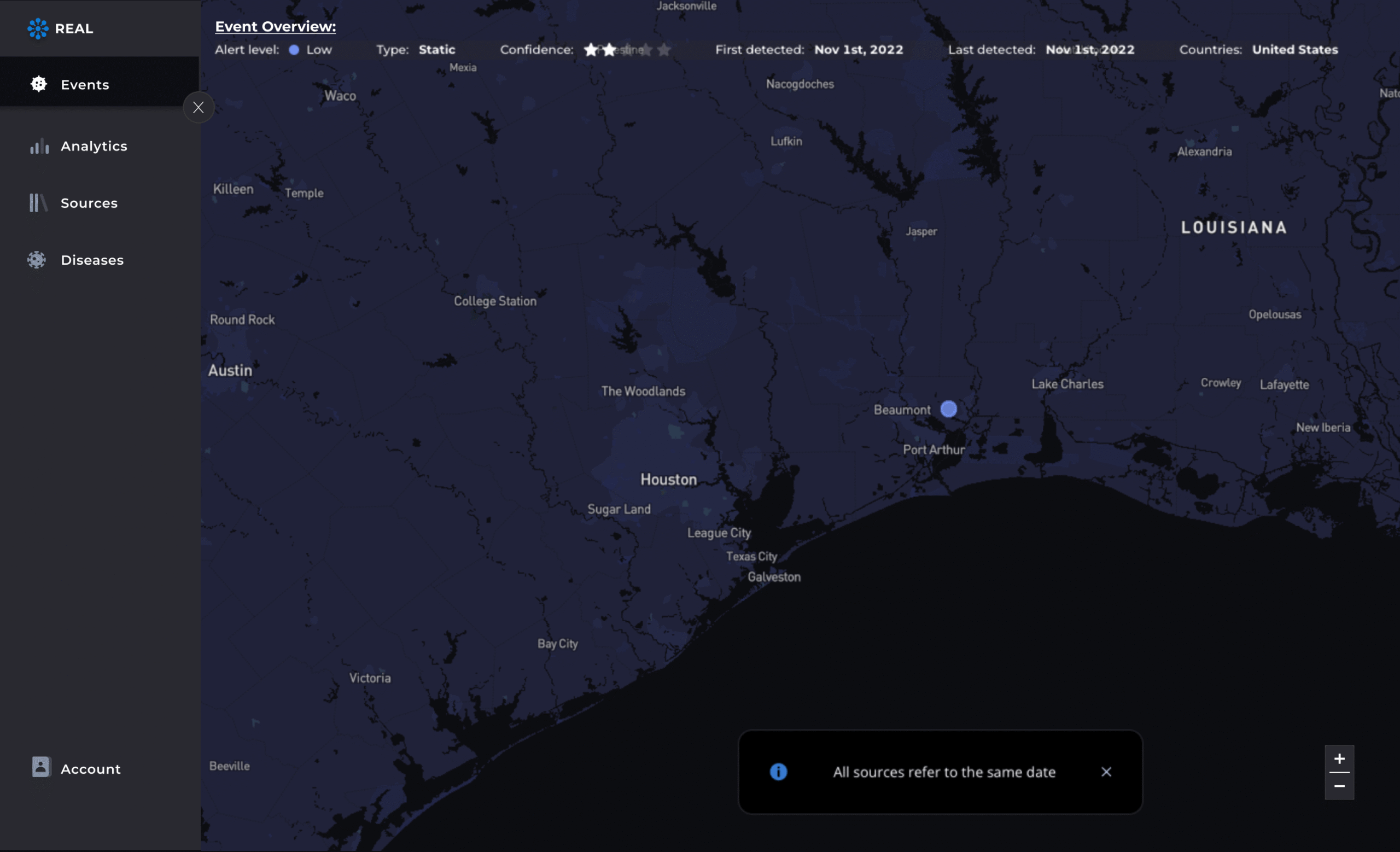
Generate reports and share insights
Export datasets, download trend visualizations, and create summary PDFs. Lifebit R.E.A.L.™ supports regulatory reporting and internal reviews—backed by full data lineage and audit trails.
Frequently asked questions
What is Lifebit R.E.A.L.™?
Lifebit R.E.A.L.™ (Real-time Evidence & Analytics Layer) is a cloud-native AI platform designed to detect, evaluate, and respond to Adverse Drug Reactions (ADRs) in real-time. It enables faster, smarter public health decision-making by automating surveillance and analytics workflows.
How does Lifebit R.E.A.L.™ support ADR surveillance?
R.E.A.L.™ continuously monitors diverse data sources, including clinical records and external media, to identify safety signals. It surfaces geographic spikes, case clusters, and risk trends via intuitive dashboards and alerts.
Who is Lifebit R.E.A.L.™ designed for?
It’s built for regulatory agencies, public health authorities, and life sciences organizations that require fast, scalable insights into drug safety and therapeutic risk.
What data sources does R.E.A.L.™ use for ADR detection?
R.E.A.L.™ integrates structured data (e.g., EHR, claims) and unstructured content (e.g., clinical notes, online reports) across federated environments—ensuring comprehensive and compliant analysis.
Can I use my own models or tools within the platform?
Yes. Lifebit R.E.A.L.™ supports full-code access, integration with custom AI/ML models, and compatibility with platforms like SageMaker and HuggingFace.
How quickly can Lifebit R.E.A.L.™ be deployed?
R.E.A.L.™ can go live within 24 hours inside secure cloud environments like AWS GovCloud, ensuring minimal setup time and immediate impact.
Is Lifebit R.E.A.L.™ compliant with data protection standards?
Absolutely. The platform complies with FedRAMP, HIPAA, and GDPR, and ensures zero data movement through federated architecture—keeping sensitive data secure and in place.
How does Lifebit R.E.A.L.™ differ from traditional pharmacovigilance tools?
Unlike legacy systems, R.E.A.L.™ combines AI-powered automation, real-time monitoring, and federated compute to deliver faster insights without compromising privacy or scale.
Work Together
Great work doesn’t happen alone. Let our experts show you how our product suite eliminates silos and unifies strategy.
Featured news and events
Talk to an expert for enterprise service solutions
Lifebit’s platform sets a new standard in real-time adverse drug reaction surveillance, empowering regulatory and research bodies with immediate insights.
Premium onboarding & workspace setup
- Enterprise-grade access control & export review
Configurable policies & role-based approvals
- 24/7 compliance & platform support