The Ultimate Guide to Real-Time Pharmacovigilance

Why Drug Safety Can’t Wait
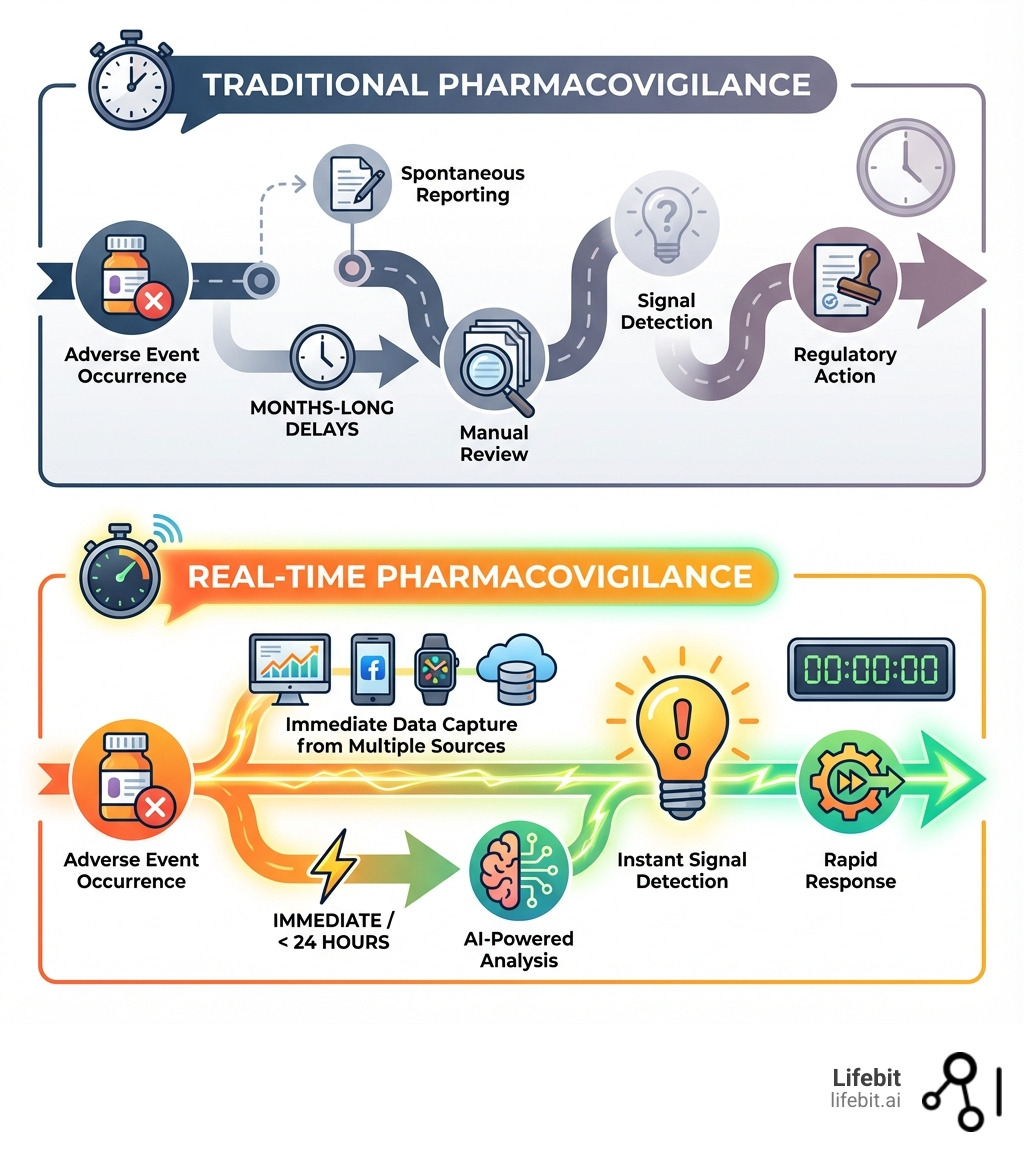
Real-time pharmacovigilance is changing how the pharmaceutical industry monitors drug safety. It enables continuous, proactive surveillance of adverse drug reactions as they happen—rather than waiting months or years for signals to emerge from traditional reporting systems.
Key differences at a glance:
- Traditional approach: Relies on spontaneous reporting systems (like FAERS), which miss 94% of adverse events due to underreporting and take months to identify safety signals.
- Real-time approach: Continuously monitors diverse data streams (EHRs, claims, social media, wearables) using AI-powered analysis for instant signal detection.
- Impact: Shifts from reactive problem-solving to proactive risk identification before widespread harm occurs.
Traditional pharmacovigilance depends on spontaneous reporting systems, but these systems miss up to 94% of adverse events. The signals they do detect can take months or even years to surface. Worse, one-third of safety issues are only found after a drug is on the market, exposing millions of patients to potential harm.
Real-time pharmacovigilance flips this model. By tapping into real-world data and applying advanced analytics, organizations can detect safety signals in under 24 hours instead of months. The FDA’s Sentinel Initiative already monitors data from over 100 million patients, while Europe’s DARWIN network covers 130 million. These systems are actively protecting public health now.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve built a federated AI platform that enables secure, compliant real-time pharmacovigilance across distributed healthcare ecosystems. Our work with public sector institutions and pharmaceutical organizations has shown me how real-time surveillance can transform patient safety outcomes.
The Shift to Proactive Safety: Understanding Real-time Pharmacovigilance
Traditional pharmacovigilance (PV) is struggling. The sheer volume and complexity of drug safety data have outpaced its reactive methods, creating significant risks for patients.
The core issue is its reactive nature. Traditional PV relies on voluntary spontaneous reports, but estimates suggest as many as 94% of adverse events go unreported. This underreporting is compounded by inherent biases, such as reporting bias (where events for newer or more notorious drugs are reported more frequently) and incomplete data, which often lacks the necessary clinical context to establish causality. The consequences of these delays can be devastating. The case of Vioxx (rofecoxib), a pain reliever withdrawn from the market in 2004, serves as a stark reminder. It was estimated to have caused tens of thousands of excess cases of heart attacks and strokes before the signal was strong enough to trigger regulatory action—a signal that could have been detected years earlier with modern real-time surveillance of electronic health records.
In fact, scientific research shows one-third of safety issues are only added to drug labels after marketing, when widespread harm may have already occurred.
Real-time pharmacovigilance represents a fundamental paradigm shift, moving from reactive analysis to proactive, continuous monitoring. It is about spotting potential issues as they emerge, rather than waiting for them to accumulate into a crisis. This approach is a necessity for safeguarding public health.
How is it different from traditional methods?
The distinction between traditional and real-time pharmacovigilance is stark.
Traditional methods (like the FDA Adverse Event Reporting System (FAERS) or WHO’s VigiBase) involve:
- Spontaneous Reporting Systems (SRS-based): Relying on voluntary submissions, which are often incomplete, biased, and suffer from massive underreporting.
- Signal Detection Delays: Manual review and batch processing mean signals can take months or years to confirm, as seen in historical cases.
- Manual Case Processing: Reviewing individual case safety reports (ICSRs) is laborious, error-prone, and consumes up to two-thirds of internal PV budgets.
- Data Gaps: Lacking the rich, longitudinal context of real-world patient journeys, making it difficult to assess comorbidities, concomitant medications, and long-term outcomes.
In contrast, real-time pharmacovigilance creates an early warning system:
- Real-Time Data Streams: Tapping into a continuous flow of diverse real-world data (RWD) from electronic health records, claims, and social media.
- Automated Analysis: AI and machine learning algorithms continuously scan these datasets, identifying potential signals instantly.
- Proactive Risk Identification: Detecting signals as they emerge allows for rapid assessment and intervention, changing PV into a life-saving strategy.
To dive deeper into the evolution of safety monitoring, you can explore more about Post-Marketing Drug Surveillance.
| Feature | Traditional Pharmacovigilance | Real-Time Pharmacovigilance |
|---|---|---|
| Data Source | Spontaneous reports, clinical trials | Real-World Data (EHRs, claims, social media, wearables) |
| Nature | Reactive, post-hoc | Proactive, continuous |
| Signal Detection | Slow (months to years), manual | Immediate (hours to days), AI-driven |
| Underreporting | High (up to 94% of adverse events missed) | Significantly reduced (potential to capture over 90%) |
| Data Volume | Limited by reporting effort | Massive, diverse, dynamic |
| Context | Often limited, incomplete | Rich, comprehensive patient context |
| Intervention Time | Delayed, after widespread exposure | Rapid, early in the product lifecycle |
| Cost Implications | High manual labor, potential for recalls/litigation | Reduced manual effort, proactive risk mitigation |
Why is this shift critical now?
The need for real-time pharmacovigilance is urgent due to several converging industry trends:
- Accelerated Drug Approvals: Regulatory pathways like the FDA’s Breakthrough Therapy designation or the EMA’s PRIME scheme allow promising drugs for serious conditions to reach patients faster. While beneficial, this means drugs are approved with smaller, more homogenous clinical trial datasets. Real-time post-marketing surveillance becomes essential to rapidly build a comprehensive safety profile in a diverse, real-world patient population.
- Complex Biologics and Digital Therapeutics (DTx): Advanced therapies like CAR-T cells, gene therapies, and monoclonal antibodies have unique and potentially severe safety profiles (e.g., cytokine release syndrome, neurotoxicity) that require close, continuous monitoring. Similarly, Digital Therapeutics (DTx) require surveillance of not just clinical effects but also risks related to software glitches, user error, and cybersecurity, which traditional PV systems are not designed to capture.
- Polypharmacy Risks: As global populations age, more patients are taking multiple medications simultaneously (polypharmacy). This dramatically increases the risk of complex and often unpredictable drug-drug interactions. Traditional systems struggle to identify these signals, but AI-powered real-time analysis of large datasets can detect patterns of harm in specific patient subgroups taking certain combinations of drugs.
- Public Health Impact and Trust: The COVID-19 pandemic demonstrated the power of real-time safety monitoring for vaccines and treatments. Rapidly analyzing data from millions of individuals allowed health authorities to quickly identify and characterize rare side effects, communicate risks transparently, and maintain public trust. As scientific research on postmarket safety outcomes shows that one-third of safety issues are only added to drug labels after marketing, the imperative for faster, more transparent detection is clear for all new medicines.
The pharmacovigilance market is growing at 6.01% annually, reaching an expected $15 billion by 2035, underscoring the industry’s recognition of this critical need for modernization.
The Engine Room: Technologies Powering the Revolution
Real-time pharmacovigilance is powered by three pillars: robust data integration, AI-driven analytics, and secure federated ecosystems.
The Role of Real-World Data (RWD)
Real-World Data (RWD) is the lifeblood of modern pharmacovigilance. It includes all health information collected outside of clinical trials, reflecting real patient experiences. The strength of real-time PV lies in its ability to synthesize insights from multiple, complementary RWD sources.
Key RWD sources include:
- Electronic Health Records (EHRs): Provide deep clinical detail, including physician notes, diagnoses, lab results, and treatment outcomes. Their richness is unparalleled for clinical investigation, but the data is often unstructured and fragmented across different systems.
- Insurance Claims Data: Offer enormous breadth, covering millions of patients with longitudinal data on prescriptions filled, diagnoses, and procedures. This makes them ideal for studying large populations, though they lack clinical granularity (e.g., lab values, actual reasons for a prescription).
- Patient Registries: Track long-term safety and effectiveness outcomes in specific patient populations (e.g., patients with a rare disease or those receiving a specific biologic). They provide high-quality, curated data for targeted research questions.
- Social Media and Online Forums: Platforms like Twitter, Reddit, and patient forums (e.g., PatientsLikeMe) can serve as early warning systems. Patients often share experiences of adverse events in their own words before they report them to a doctor. While this data is noisy and requires sophisticated NLP to analyze, its real-time nature is a key advantage.
- Wearables and the Internet of Medical Things (IoMT): Devices like smartwatches, continuous glucose monitors, and smart inhalers capture continuous physiological data (heart rate, activity levels, sleep patterns, blood oxygen). This objective, high-frequency data can reveal subtle signs of an ADR that might otherwise go unnoticed.
- Genomic Data: Linking clinical data with genomic information allows researchers to identify genetic markers that may predispose certain individuals to adverse reactions, paving the way for personalized safety predictions.
The scale of modern RWD systems is impressive. The FDA’s Sentinel Initiative created a distributed network spanning over 100 million patients in the USA. In Europe, the EMA’s DARWIN initiative covers 130 million patients across 40 data partners. These massive datasets are the foundation for rapid safety surveillance.
How AI and Machine Learning Are Game-Changers
AI and Machine Learning (ML) are essential to analyze the volume, velocity, and variety of RWD, finding signals impossible for humans to detect manually.
- Natural Language Processing (NLP): This technology is critical for unlocking the 80% of healthcare data that is unstructured text. NLP models can read and interpret physicians’ notes in EHRs, patient posts on social media, and scientific literature to extract key information like symptoms, drug names, dosages, timelines, and patient sentiment, turning narrative chaos into structured, actionable data.
- Predictive Modeling: AI models can be trained on historical data to predict which patients are at higher risk of developing certain ADRs based on their clinical profile, comorbidities, and concomitant medications. This allows for proactive interventions in high-risk populations.
- Advanced Signal Detection: While traditional methods use simple disproportionality analysis (e.g., calculating reporting odds ratios), AI algorithms can analyze complex, multi-modal data to identify subtle deviations from expected patterns that could indicate a new safety signal. These models can account for confounding factors and detect complex interactions that older methods miss.
- Automated Case Processing: AI automates highly repetitive tasks like data extraction from source documents, MedDRA coding for adverse events, and case deduplication. This significantly reduces manual labor, freeing up human experts to focus on complex signal validation and risk assessment. The Uppsala Monitoring Centre (UMC) already uses ML tools like vigiMatch and vigiRank to improve case and signal processing.
- Causal Inference Models: A key challenge in RWD is distinguishing correlation from causation. Advanced statistical and ML techniques, such as propensity score matching and instrumental variable analysis, are used to emulate the properties of a randomized controlled trial. These causal inference methods help determine whether a drug actually caused an adverse event or was merely associated with it due to other factors (confounding by indication).
For a comprehensive dive into the role of AI, explore An AI Pharmacovigilance Guide for 2025.
The Power of Federated Ecosystems
Analyzing global RWD presents major data privacy and security challenges. Patient data is sensitive, protected by strict regulations like GDPR and HIPAA, and often cannot leave the hospital or institution where it was generated. Federated ecosystems, like the one we’ve built at Lifebit, are the solution.
- Data Privacy by Design (GDPR, HIPAA): Federated learning is a privacy-preserving approach. Instead of centralizing sensitive data, the analytical model is securely sent to the data. The model is trained locally on the data behind the institution’s firewall. Raw patient data never moves or is exposed.
- Federated Learning Workflow: The process involves distributing a global AI model to multiple data partners. Each partner trains the model on their local data. Only the updated, non-sensitive model parameters (aggregated mathematical insights) are sent back to a central coordinator. These insights are then aggregated to create an improved global model, which can be redistributed for further training. This iterative process allows a model to learn from diverse, global datasets without ever centralizing them.
- Analyzing Data Without Moving It: This approach breaks down data silos, enabling large-scale global safety studies that would otherwise be impossible due to privacy, security, or data sovereignty concerns. It is vital for detecting rare ADRs, which requires pooling statistical power from multiple international datasets.
- Secure Collaboration in Trusted Research Environments (TREs): Federated analysis often takes place within TREs. These are highly secure computing environments that provide approved researchers with access to data for analysis while maintaining strict controls. The TRE ensures that only approved analyses are run and only non-identifiable, aggregate results can be exported, providing an additional layer of governance and security.
Our federated AI platform, with its Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL) components, is designed for this purpose. Our R.E.A.L. (Real-time Evidence & Analytics Layer) ensures we can conduct Real-time Adverse Drug Reaction Surveillance securely across hybrid data ecosystems.
Benefits vs. Problems: The Business Case and Implementation Path
The shift to real-time pharmacovigilance offers transformative benefits but also presents implementation challenges. Understanding both is key to a successful strategy.

The Benefits of Adopting a real-time pharmacovigilance Strategy
Adopting a real-time pharmacovigilance system delivers far-reaching advantages:
- Improved Patient Safety: This is the primary benefit. By detecting adverse events in hours or days instead of months, organizations can intervene faster to prevent widespread harm. The ability to capture a much higher percentage of events (potentially over 90% compared to just 6% in traditional systems) provides a far more accurate picture of a drug’s true safety profile.
- Proactive Risk Management: Real-time systems enable a shift from reacting to crises to proactively identifying and managing risks. This allows for earlier, more targeted interventions, such as updating drug labels, issuing physician advisories, or implementing risk evaluation and mitigation strategies (REMS) before a problem escalates.
- Reduced Healthcare and Corporate Costs: Adverse drug reactions are a significant financial burden, costing healthcare systems billions annually in hospitalizations and additional treatments. By preventing ADRs, real-time PV reduces these costs. For pharmaceutical companies, it mitigates the immense financial risk of late-stage drug recalls, litigation, and brand damage.
- Strengthened Regulatory Relationships: Providing regulators with timely, high-quality, and transparent RWD demonstrates a commitment to patient safety. This can build stronger, more collaborative relationships and potentially streamline regulatory processes, as regulators gain confidence in the post-marketing surveillance plan.
- Enhanced Drug Development and Commercialization: Real-time safety insights can be fed back into the R&D pipeline to inform future drug design. These insights can also identify specific patient populations where a drug has a superior benefit-risk profile, enabling more targeted commercial strategies and personalized medicine approaches.
- Increased Operational Efficiency: Automating routine tasks like data collection, case processing, and literature screening can reduce manual workloads by over 50%. This frees up highly skilled pharmacovigilance experts to focus on high-value activities like signal validation, causal assessment, and strategic risk management.
Overcoming the Challenges of Implementing real-time pharmacovigilance
While the benefits are compelling, implementation is a complex journey. Key challenges include:
- Data Quality and Standardization: RWD is inherently messy, heterogeneous, and collected for clinical care, not research. To make it usable for analysis, it must be standardized. This is a major hurdle, but it is being addressed by the adoption of Common Data Models (CDMs) like the OMOP CDM, which transforms data from diverse sources into a standard structure and terminology.
- Interoperability and Integration: Healthcare data is often locked in siloed systems (EHRs, claims databases, registries) that do not speak to each other. Achieving seamless data integration requires sophisticated technical solutions and adherence to interoperability standards like HL7 FHIR (Fast Healthcare Interoperability Resources), which provides a modern API for exchanging healthcare information.
- Data Privacy and Security: Handling vast amounts of sensitive patient data demands uncompromising adherence to global regulations like GDPR and HIPAA. A federated approach, where data is analyzed in place without being moved, is the gold standard for ensuring compliance and maintaining patient trust.
- High Initial Investment: Transitioning to real-time systems requires significant upfront investment in technology infrastructure, data access licenses, specialized software, and building a skilled team. However, this should be viewed as a long-term investment that yields substantial returns through risk reduction and operational efficiency.
- Specialized Talent Gap: There is a global shortage of professionals who possess the unique blend of skills required for real-time PV: expertise in pharmacovigilance, data science, AI/ML, and regulatory affairs. Building a successful team often requires a combination of upskilling internal talent, strategic hiring, and partnering with specialized technology providers.
- AI Model Validation and Governance: For AI-driven signals to be trusted, the models must be rigorously validated. This involves demonstrating their accuracy, precision, and recall on historical and external datasets. Organizations must also establish strong governance frameworks for monitoring models over time to detect and correct for “model drift,” where performance degrades as real-world data patterns change.
- Explainable AI (XAI) for Regulatory Acceptance: AI models can often be a \”black box,\” making it difficult for regulators and clinicians to trust their outputs. Explainable AI (XAI) techniques are vital for overcoming this barrier. Methods like LIME (Local Interpretable Model-agnostic Explanations) and SHAP (SHapley Additive exPlanations) provide insights into why a model flagged a particular signal, making the process transparent and defensible.
The Regulatory Horizon and Future Innovations
The regulatory landscape is evolving to support real-time pharmacovigilance, with leading agencies around the world embracing RWD and AI to foster innovation in drug safety.
How Regulators are Paving the Way
Regulators are not just permitting but actively building the infrastructure for modern drug safety:
- FDA’s Sentinel System (USA): A pioneering distributed data network monitoring over 100 million patients, Sentinel has become a cornerstone of the FDA’s surveillance efforts. It enables the agency to run rapid safety analyses on approved drugs and vaccines, moving from passive observation to proactive surveillance.
- EMA’s DARWIN EU (Europe): This federated network gives regulators and researchers access to real-world evidence from an estimated 130 million patients across Europe. It was recently used to investigate a potential safety signal for semaglutide, with the EMA reviewing and concurring with the RWD findings, demonstrating its real-world utility.
- Global Initiatives (WHO, PMDA): The WHO is advancing global capabilities with tools like VigiFlow and VigiMobile to streamline adverse event reporting, while VigiLyze uses analytics to help member countries establish real-time monitoring. In Asia, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) has established the MID-NET database, a hospital-based network for post-marketing surveillance, reflecting a global trend towards leveraging RWD.
- Real-World Evidence (RWE) Frameworks: Both the FDA and EMA have published formal guidance documents on the use of RWE to support regulatory decisions. This signals a clear shift: real-time pharmacovigilance is no longer a theoretical concept but is becoming an integral and expected part of the regulatory framework for ensuring drug safety.
What’s Next: Emerging Trends in Drug Safety
The evolution of real-time pharmacovigilance continues, driven by cutting-edge science and technology:
- Pharmacogenomics Integration: The future is personal. By integrating genomic data into RWD platforms, we can move from population-level risk assessment to predicting individual patient risk. For example, knowing that patients with the HLA-B*5701 allele have a high risk of a hypersensitivity reaction to the HIV drug abacavir allows for pre-treatment screening, a model for future personalized safety.
- Advanced Therapy Medicinal Products (ATMPs): The long-term safety of revolutionary gene and cell therapies is largely unknown. Real-time monitoring through linked registries and EHR data will be crucial for tracking long-term risks such as immunogenicity, off-target effects, and secondary malignancies, ensuring the safety of these transformative treatments.
- Ecopharmacovigilance: Drug safety is expanding beyond the patient to the planet. This emerging field monitors the environmental impact of pharmaceutical residues in water and soil and their effects on ecosystems. Real-time monitoring of environmental data will become a new frontier in responsible pharmaceutical stewardship.
- Internet of Medical Things (IoMT) and Digital Biomarkers: The data stream from connected medical devices—such as smart inhalers tracking usage patterns, smartwatches detecting atrial fibrillation, or continuous glucose monitors—will provide an unprecedented flow of objective, real-time physiological data. This will enable the detection of subtle ADRs and the development of ‘digital biomarkers’ for safety events.
- Quantum Computing: While still in its infancy, quantum computing holds the promise of solving problems that are currently intractable. In pharmacovigilance, it could one day be used to simulate complex drug-drug-gene interactions at a molecular level, predicting adverse events with incredible accuracy before they ever occur.
- Blockchain for Data Integrity and Traceability: In a complex ecosystem of data providers, researchers, and regulators, ensuring data integrity is paramount. Blockchain technology could provide an immutable, transparent, and auditable trail for pharmacovigilance data, tracking its provenance and ensuring that analyses are based on trustworthy information.
For a deeper look into how these advanced technologies are shaping the future, explore A deeper look at Drug Safety AI.
Frequently Asked Questions about Real-Time Pharmacovigilance
How can an organization transition from traditional to real-time PV?
A phased approach is the most effective way to transition:
- Start with a Pilot Project: Select a specific drug or therapeutic area to test your approach, refine processes, and demonstrate value on a manageable scale.
- Identify Key RWD Sources: Determine which real-world data sources (EHRs, claims, etc.) are most relevant and accessible for your pilot.
- Invest in a Scalable Technology Platform: A secure, scalable, and federated platform is essential for data integration, analytics, and collaboration.
- Build a Multi-Disciplinary Team: Success requires collaboration between pharmacovigilance experts, data scientists, and AI/ML engineers.
- Develop a Phased Implementation Roadmap: Use learnings from the pilot to create a clear roadmap for expanding real-time capabilities across your portfolio.
What is the typical return on investment (ROI)?
The ROI is measured in both financial and strategic benefits:
- Reduced Costs: Avoid expensive drug recalls and litigation while optimizing resources through automation. The growing $15 billion pharmacovigilance market highlights the financial incentive for efficiency.
- Improved Operational Efficiency: AI can reduce literature surveillance times by 35-50% and screening times by up to 80%, freeing up expert resources.
- Faster Market Access: Demonstrating superior safety monitoring can improve regulatory relationships and provide a competitive advantage.
- Improved Brand Reputation: Proactive safety management builds trust with patients, providers, and regulators.
- Better Patient Outcomes: The ultimate ROI is the prevention of adverse events and the improvement of patient health.
How do you ensure the quality of AI-driven safety signals?
Quality is ensured through a multi-layered approach:
- Rigorous Model Validation: Models are extensively tested against known datasets to ensure their accuracy, sensitivity, and specificity.
- Explainable AI (XAI) Techniques: We use XAI to provide transparency into how a model reached a conclusion, building trust and facilitating regulatory review.
- Human-in-the-Loop Oversight: AI is a tool, not a replacement for expertise. Human pharmacovigilance experts review and validate AI-generated signals for critical decisions.
- Triangulating Signals Across Multiple Data Sources: We increase confidence in a signal’s validity by cross-referencing it across diverse RWD sources like EHRs, claims, and social media.
Conclusion: Securing the Future of Patient Safety
As new therapies and accelerated approvals redefine the pharmaceutical landscape, the shift to real-time pharmacovigilance is no longer an option—it is a necessity. It moves drug safety from a reactive task to a proactive, life-saving strategy that protects patients and builds trust.
Traditional methods are too slow. In contrast, real-time pharmacovigilance uses RWD, AI, and federated ecosystems to detect signals in hours, improving safety, increasing efficiency, and reducing costs. While challenges like data quality exist, regulators are paving the way with initiatives like the FDA’s Sentinel and EMA’s DARWIN. Future trends like pharmacogenomics and the IoMT will further improve our capabilities.
At Lifebit, we believe technology is the key to open uping this potential globally. Our federated AI platform empowers organizations to implement secure and scalable real-time surveillance, protecting patient data while generating critical insights. We are building the intelligent, connected systems that will define the future of drug safety.
The future of patient safety depends on our collective ability to accept this paradigm shift. Let’s work together to make real-time pharmacovigilance the new standard. Discover how to build your real-time adverse drug reaction surveillance system and join us in securing a safer tomorrow for patients worldwide.

