Unlocking Tomorrow: How AI Transforms Life Sciences

AI for Life Sciences: Cut Discovery Time by 33% and Hit 3X ROI
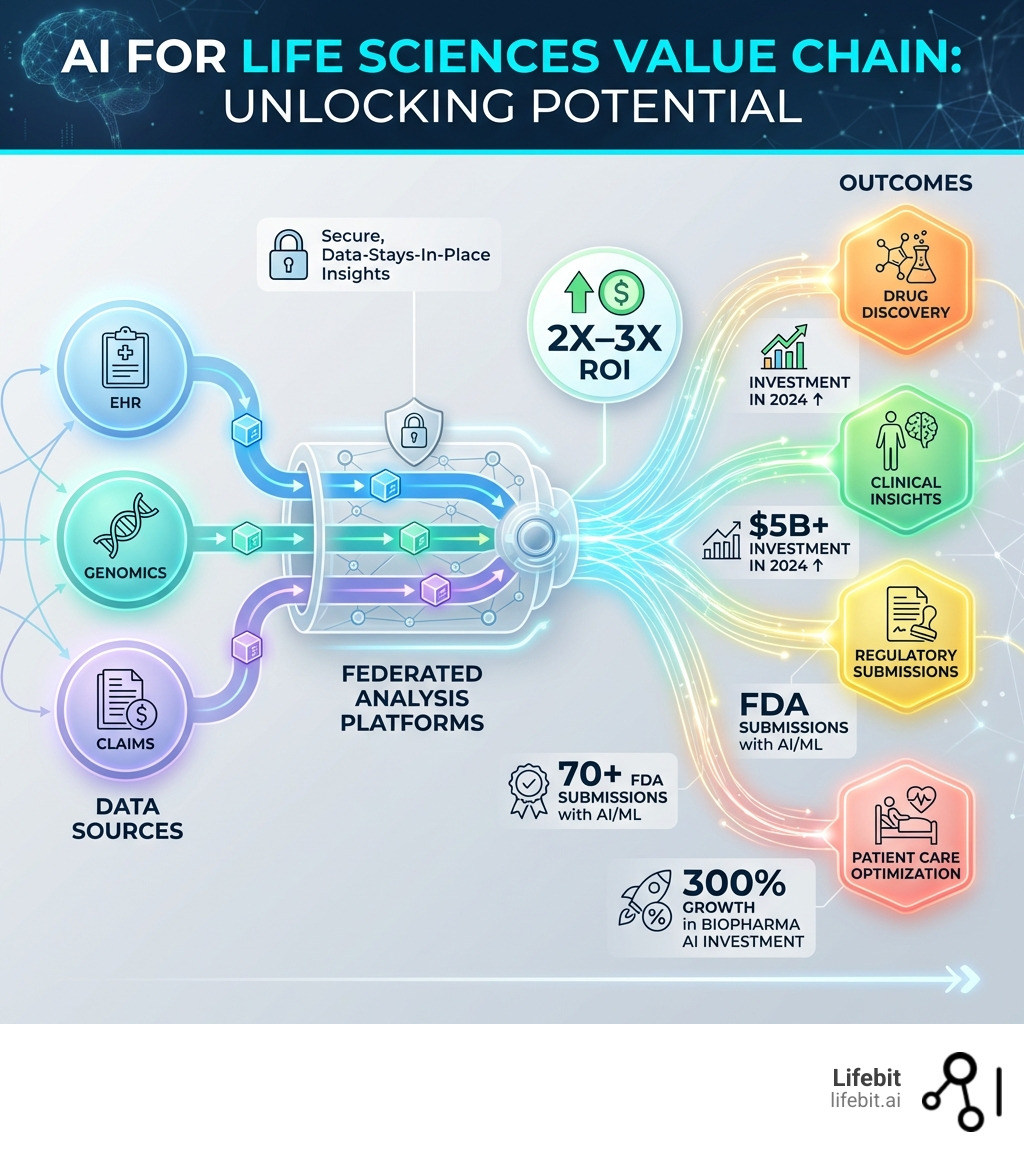
AI for life sciences is no longer experimental—it’s essential for commercial success. Organizations embedding AI across drug findy, clinical trials, diagnostics, and patient care are achieving 2X–3X ROI while moving from ambition to enterprise-scale execution. In the current competitive landscape, the ability to harness large-scale biological data is the primary differentiator between market leaders and those struggling with R&D stagnation.
Key applications changing the industry:
- Drug Findy: AI accelerates target identification, molecular design, and compound selection—cutting development time by up to a third. By utilizing deep learning models to predict binding affinities, researchers can bypass thousands of physical lab tests, focusing only on the most promising candidates.
- Clinical Trials: Real-time patient monitoring, predictive recruitment, and automated documentation reduce timelines and costs. AI algorithms can now analyze electronic health records (EHR) to identify patients who meet complex inclusion criteria in seconds, a process that previously took months.
- Diagnostics & Imaging: AI-powered tools detect patterns in X-rays, MRIs, and pathology slides with precision that rivals or exceeds human experts. These systems are particularly effective in early-stage oncology, where subtle cellular changes are often missed by the naked eye.
- Administrative Automation: AI scribes and workflow tools free clinicians from paperwork, capturing 42% of all AI deals in 2025. This shift is critical for addressing the global healthcare worker shortage by reducing burnout and increasing patient throughput.
- Regulatory Compliance: Over 70 FDA investigational new drug applications now involve AI or machine learning. Regulatory bodies are increasingly expecting sophisticated data governance and transparency in how these models are trained and validated.
The numbers tell the story. Over $5 billion flowed into biopharma AI companies in 2024 alone—surpassing 2021 totals by nearly $2 billion. AI-focused startups secured 60% of all digital health funding in Q1 2025, raising $3.2 billion. Investment in biopharma AI jumped 300% since 2023. This capital is being funneled into “platform-first” companies that don’t just solve one problem, but provide an end-to-end infrastructure for biological intelligence.
But scaling AI isn’t just about funding. It’s about overcoming barriers: siloed data, regulatory uncertainty, algorithmic bias, and the challenge of integrating AI into existing research infrastructure. Organizations that succeed are those that build secure, federated environments where data stays in place while insights move freely. This “data-centric” approach ensures that privacy is maintained while the power of global datasets is fully realized.
I’m Maria Chatzou Dunford, CEO and Co-founder of Lifebit, where we’ve spent over 15 years building federated platforms that enable AI for life sciences across genomics, clinical trials, and drug findy. Our work powers secure, compliant analytics for global pharma and public sector institutions—turning data into evidence without moving it. We believe that the future of medicine lies in the ability to analyze data where it lives, respecting sovereignty while accelerating discovery.
AI for life sciences word roundup:
- health data integration
- data integrity in health information systems
- Can you list firms that specialize in clinical trial data integration platforms?
AI for Life Sciences: Stop Wasting Decades and Fix Drug Discovery
The landscape of AI for life sciences has shifted from “what if” to “what’s next.” Today, we aren’t just using algorithms to sort through spreadsheets; we are using them to simulate the very building blocks of biology. Whether it’s predicting how a protein folds in a lab in London or monitoring a patient’s heart rate in New York, AI is the engine driving these breakthroughs. The convergence of high-performance computing and massive biological datasets has created a “perfect storm” for innovation.
Currently, the primary applications of AI span the entire value chain. In research, AI for medical research is being used to analyze massive multi-omic datasets that would take human researchers decades to parse. This includes transcriptomics, proteomics, and metabolomics, providing a holistic view of disease mechanisms that was previously impossible to visualize. In the clinical setting, AI-powered tools are moving from the back office to the bedside, providing real-time insights that save lives by predicting patient deterioration hours before it occurs.
For those interested in the deep technical shifts, you can explore the scientific research on AI in Life Sciences which highlights how theoretical advances in Graph Neural Networks (GNNs) and Transformer models are becoming practical realities in molecular modeling.
Changing Drug Findy with AI for Life Sciences
The traditional drug findy process is famously slow and expensive—often cited as costing over $1 billion and taking over a decade. We like to call this the “Eroom’s Law” problem (Moore’s Law in reverse), where drug discovery becomes slower and more expensive over time despite improvements in technology. AI is finally breaking this cycle by introducing efficiency at every stage of the pipeline.
One of the most significant successes has been in protein structure prediction. Tools like AlphaFold have revolutionized our understanding of biology by predicting the 3D shapes of proteins with incredible accuracy. This is a game-changer because a protein’s shape determines its function. By knowing the shape, we can design drugs that fit like a key into a lock, reducing the “off-target” effects that often lead to clinical trial failure.
Key successes in this area include:
- Target Identification: Using AI to find the biological “bullseye” for a disease. By analyzing genetic variants across millions of individuals, AI can pinpoint the exact genes responsible for disease progression.
- Molecular Design: Generative models that “dream up” new molecules with specific properties. Instead of searching for a needle in a haystack, AI allows us to build the needle from scratch.
- Lead Optimization: Refining drug candidates to be more effective and less toxic before they ever reach a human. AI can predict the ADME (Absorption, Distribution, Metabolism, and Excretion) profile of a molecule with high precision.
- De-novo Design: Creating entirely new therapeutic modalities, such as macrocycles or complex biologics, that traditional chemistry could not easily access.
Organizations are increasingly unlocking the potential of AI in drug discovery to identify biological targets that cause disease and select potential drug candidates that interact with them. In fact, as of 2024, over 70 investigational new drug applications submitted to the U.S. FDA involve AI or machine learning in some capacity. This regulatory acceptance is a watershed moment for the industry.
Streamlining Clinical Trials and Diagnostics
If drug findy is about finding the right medicine, clinical trials are about finding the right people and the right data. We know that trial delays often stem from poor patient recruitment and high dropout rates. AI solves this by analyzing real-world data to identify patient populations most likely to benefit from a new treatment, thereby increasing the probability of success.
In the clinic, AI is changing:
- Patient Recruitment: Predictive models identify eligible participants faster, ensuring trials stay on schedule. This includes analyzing social media, EHRs, and genomic databases to find rare disease patients who might otherwise be missed.
- Real-Time Monitoring: Wearables and AI sensors monitor study subjects 24/7, catching adverse events before they become dangerous. This continuous data stream provides a much richer picture of drug efficacy than periodic clinic visits.
- Predictive Diagnostics: AI models in medical imaging detect abnormalities in X-rays and MRIs with superhuman precision, often acting as a “second set of eyes” for radiologists. In pathology, AI can quantify tumor-infiltrating lymphocytes, a key metric for immunotherapy response.
- Administrative Burden: Tools like “AI scribes” are reducing the paperwork that burns out clinicians. Globally, investors have raised over $1.6 billion for these workflow tools in 2024 alone, recognizing that the “human element” of healthcare needs technological support to remain sustainable.
By using AI clinical trials, we can move toward decentralized models that make research more inclusive and efficient. This allows patients from diverse geographic and socioeconomic backgrounds to participate in trials from their own homes, leading to more representative and robust data.
AI for Life Sciences: Break Data Silos and Bypass Privacy Risks
Despite the excitement, it’s not all smooth sailing. If you’ve ever tried to get two different hospital databases to “talk” to each other, you know the pain of data silos. These silos are the single biggest barrier to implementing AI for life sciences. Data is often trapped in legacy systems, formatted in incompatible ways, and protected by strict legal frameworks that make sharing nearly impossible.
The most significant challenges include:
- Data Quality and Accessibility: AI is only as good as the data it’s trained on. Fragmented, unformatted data is useless. Without standardized ontologies (like SNOMED or LOINC), AI models struggle to make sense of disparate records.
- Regulatory Problems: Rules like GDPR in Europe and HIPAA in the USA are essential for privacy but can make data sharing difficult. The risk of heavy fines and reputational damage often leads organizations to be overly cautious, locking down data that could be used for life-saving research.
- Ethical Concerns: We must ensure that AI doesn’t learn the biases present in historical medical data, which could lead to health disparities. For example, if a model is trained primarily on data from one ethnic group, its diagnostic accuracy may suffer when applied to others.
- Interoperability: The lack of a unified data standard across the global healthcare ecosystem means that even when data is shared, it often requires massive manual effort to clean and harmonize.
Navigating Regulatory and Compliance Challenges
Compliance isn’t just a “check the box” activity; it’s the foundation of trust in healthcare. Regulatory bodies like the FDA and Health Canada are catching up, releasing guidance on how to use machine learning safely. The introduction of the EU AI Act also adds a new layer of complexity, requiring high-risk AI systems in healthcare to meet stringent transparency and safety standards.
For instance, regulatory compliance RWE is now a major focus for biopharma. Developers must prove that their AI models are transparent, reproducible, and secure. This involves rigorous clinical data governance to ensure that patient privacy is protected at every step of the research journey. Organizations must implement “Privacy by Design,” ensuring that data protection is baked into the technology from the very first line of code.
Solving the Data Integration Crisis
To truly scale AI, we need to move away from moving data. Every time data is moved, it risks being lost, hacked, or corrupted. Furthermore, the sheer volume of genomic data—often petabytes in size—makes traditional data transfer impractical and prohibitively expensive. This is where federated governance comes in.
At Lifebit, we advocate for a “data stays, insights move” approach. By using a federated data platform, researchers can analyze multi-omic data (genomics, proteomics, etc.) across different continents without the data ever leaving its secure home. This solves the harmonization problem by creating a trusted data lakehouse where disparate datasets are standardized and made “AI-ready.” This architecture allows for “federated learning,” where an AI model is trained across multiple decentralized servers holding local data samples, without exchanging them. This preserves privacy while allowing the model to learn from a much larger and more diverse dataset than any single institution could provide.
2025 AI for Life Sciences: Scale from Prototype to Enterprise ROI
The money is following the results. As we’ve seen, investment in biopharma AI has skyrocketed. But where is that money going? It’s moving away from “cool prototypes” and toward enterprise-scale execution. The industry is tired of “pilot purgatory,” where promising AI projects fail to make it into production. Today, the focus is on building robust, scalable systems that can support thousands of researchers simultaneously.
Investors are looking for platforms that can handle the “boring” but essential parts of AI: data ingestion, cleaning, and compliance. Startups in the USA secured $3.2 billion in Q1 2025 alone, with a massive focus on clinical decision support and patient diagnostics. We are also seeing a rise in M&A activity, as traditional pharma giants acquire AI-native biotech firms to bolster their internal pipelines.
2023 vs. 2025: The AI Funding Explosion
| Metric | 2023 | 2025 (Projected/YTD) |
|---|---|---|
| Biopharma AI Investment | ~$1.2B | $5B+ |
| Provider-Centric AI Tools | $390M | $1.6B+ |
| Digital Health AI Funding % | 41% | 60% |
| ROI for AI Adopters | 1X-1.5X | 2X-3X |
| Average Time Saved in R&D | 10% | 33% |
| FDA AI/ML Submissions | 45 | 70+ |
Scaling AI for Life Sciences from Ambition to Enterprise
Scaling AI requires more than just a bigger budget; it requires a strategy that aligns technology with business outcomes. We see successful organizations following a specific playbook:
- Build the Infrastructure: Move from legacy warehouses to application of data lakehouses in life sciences. A lakehouse combines the flexibility of a data lake with the management of a data warehouse, providing a single source of truth for both structured and unstructured data.
- Empower the Scientists: Use AI for Nextflow and other workflow tools to automate repetitive tasks. By automating the bioinformatic pipelines, scientists can spend more time on hypothesis generation and less on data plumbing.
- Focus on ROI: Prioritize use cases that deliver clear value, such as reducing trial timelines or improving drug safety. This “value-first” approach helps build internal buy-in and secures continued funding for AI initiatives.
- Change Management: Recognize that AI adoption is as much a cultural shift as a technical one. Training staff to work alongside AI tools is essential for maximizing the technology’s impact.
By adopting an enterprise data platform, companies can ensure that AI is embedded in every department, from early-stage discovery to post-market surveillance, rather than being confined to a lonely “innovation lab.”
AI for Life Sciences: Use Generative AI to Design Molecules in Real-Time
The “GenAI revolution” is just getting started. While the last decade was about analyzing data, the next decade is about generating it. Generative AI is helping scientists design better molecules and even simulate how a drug might affect a specific patient’s biology—a concept known as the “Digital Twin.” These twins allow researchers to run thousands of “in-silico” trials before a single patient is ever enrolled, identifying potential safety issues early.
Generative models, such as Variational Autoencoders (VAEs) and Generative Adversarial Networks (GANs), are being used to explore the vast “chemical space” of potential drugs, which is estimated to contain $10^{60}$ molecules. Human chemists can only explore a tiny fraction of this space; AI can navigate it systematically to find optimized structures.
Future trends we are watching include:
- Personalized Care: Using AI for precision medicine to tailor treatments to an individual’s genetic makeup. This is particularly transformative in rare diseases, where “N-of-1” trials are becoming a reality.
- Real-Time Evidence: Moving from static reports to real-time healthcare analytics that inform clinical decisions as they happen. Imagine a surgeon receiving real-time AI guidance on tumor margins during an operation.
- Multi-Omic Integration: Combining DNA, RNA, and protein data to get a 360-degree view of human health. This holistic approach allows us to understand not just the genetic blueprint, but how that blueprint is being executed in the body.
- Targeted Therapies: Using AI-powered target identification to find cures for rare diseases that were previously thought untreatable. AI can identify “druggable” pockets on proteins that were previously considered “undruggable.”
- Synthetic Data Generation: Creating high-fidelity synthetic patient data to train models when real-world data is scarce or too sensitive to share. This can accelerate AI development while maintaining 100% patient privacy.
We are entering an era where healthcare is proactive, not reactive. Instead of treating a disease after it appears, we will use predictive modeling to prevent it from ever happening. This shift from “sick care” to true “health care” is the ultimate promise of AI in the life sciences.
AI for Life Sciences: Your Questions on ROI and Ethics Answered
What are the biggest ethical risks of AI in healthcare?
The primary ethical risks involve algorithmic bias and patient privacy. If the data used to train an AI doesn’t include diverse populations, the AI’s recommendations might not be accurate for everyone, potentially exacerbating existing health disparities. Furthermore, ensuring preserving patient data privacy and security is paramount. We must also consider informed consent—do patients know how their data is being used by an algorithm? There is also the risk of “automation bias,” where clinicians might follow an AI’s recommendation even when their professional judgment suggests otherwise. Establishing clear “human-in-the-loop” protocols is essential to mitigate these risks.
How does AI reduce administrative burdens for clinicians?
Clinicians spend up to 50% of their time on paperwork, a major contributor to the global burnout crisis. AI-powered tools like “AI scribes” use Natural Language Processing (NLP) to listen to patient consultations and automatically generate clinical notes, which the doctor can then review and sign. Other tools automate patient scheduling, diagnostic report summarization, and insurance form completion. By handling these repetitive tasks, AI allows doctors to spend more time with patients and less time with their keyboards, improving both the provider and patient experience.
What is the ROI of enterprise-scale AI adoption?
Current data shows that organizations moving to enterprise-scale execution are achieving 2X–3X ROI. This comes from several key areas: faster time-to-market for new drugs (which can be worth millions per day in patent life), reduced waste in R&D by failing “bad” candidates earlier, and improved patient outcomes which lead to higher value-based care reimbursements. By using AI-driven insights, companies can fail faster in the lab and succeed faster in the clinic. Additionally, AI-driven supply chain optimization can significantly reduce the costs of manufacturing and distributing complex biologics.
How does federated learning differ from traditional data sharing?
In traditional data sharing, data is copied from various sources and moved to a central repository. This creates security risks and often violates data sovereignty laws. In federated learning, the data stays at its original location (e.g., a hospital or a research center). Instead of moving the data, the AI model is sent to the data. The model “learns” from the local data and then sends only the updated model parameters (not the data itself) back to a central server. This allows for the creation of powerful, global AI models while ensuring that sensitive patient data never leaves its secure environment. It is the only viable way to build AI on a global scale while remaining compliant with local privacy regulations.
AI for Life Sciences: Stop Losing to Silos and Launch Your Platform Today
The transition from AI ambition to enterprise-scale execution is the defining challenge of our time in the life sciences. We’ve seen that the technology is ready, the investment is there, and the ROI is proven. The only thing left is the execution. Organizations that continue to rely on fragmented data and manual processes will find themselves increasingly unable to compete with AI-native firms that can iterate and discover at ten times the speed.
At Lifebit, we are proud to be at the heart of this change. Our next-generation federated AI platform enables secure, real-time access to global biomedical and multi-omic data. We help organizations across five continents—from London to Singapore—solve the data integration crisis through components like our Trusted Research Environment (TRE) and Trusted Data Lakehouse (TDL). Our platform is designed to be “cloud-agnostic,” meaning it can run on AWS, Azure, or Google Cloud, providing the flexibility that modern global enterprises require.
Our R.E.A.L. (Real-time Evidence & Analytics Layer) ensures that biopharma, governments, and public health agencies have the AI-powered research tools they need to bring breakthroughs to market faster and safer. We are not just providing a tool; we are providing the foundation for the next century of medical innovation. The era of data silos is over; the era of federated intelligence has begun.
Stop wasting time on data silos. Start open uping tomorrow.

