In Depth Guide to Federated Learning in Precision Medicine

Why Federated Learning Is Changing Precision Medicine
Federated learning is revolutionizing precision medicine by enabling healthcare institutions to train powerful AI models on distributed datasets without sharing sensitive patient information. This privacy-preserving approach breaks down data silos, allowing multiple hospitals to collaborate on research without centralizing records. The results are clear: federated models have matched or surpassed centralized models in 15 out of 25 recent studies.
Today’s most promising medical research requires large, diverse datasets, but this data is often locked away by privacy laws and institutional boundaries. A single breast cancer study across four hospitals once took a researcher six years to coordinate—by which time the data was obsolete. Federated learning eliminates these delays.
Instead of moving data, federated learning brings the computation to the data. Each institution trains AI models locally and shares only the encrypted model parameters—not the patient data—with a central server for aggregation. This allows organizations to collaborate at scale while maintaining complete control. Federated models have already achieved 92% accuracy compared to 89% for centralized methods, with lower latency and greater resilience. They are accelerating research on rare diseases, improving cancer diagnostics, and powering personalized treatment plans that were previously impossible.
I’m Dr. Maria Chatzou Dunford, CEO and Co-founder of Lifebit. With over 15 years in computational biology and AI, I’ve seen how breaking down data barriers enables breakthroughs. At Lifebit, we’ve pioneered federated genomics platforms that power secure collaboration for pharmaceutical and public sector partners worldwide.
Key terms to learn:
The Data Dilemma: Why Precision Medicine Is Stuck
Precision medicine promises treatments custom to your unique genetic makeup, lifestyle, and environment. The vision is a cancer therapy designed for your tumor’s specific molecular profile or a medication dosed perfectly for your metabolism. But this vision is stalled by a massive data problem.
The fuel for precision medicine is vast, diverse data, and most of it is locked away. It’s scattered across thousands of hospitals and clinics in isolated data silos, unable to communicate. Each institution uses its own Electronic Health Records (EHRs) with different formats and standards. A patient’s data is fragmented across multiple systems, making it impossible for researchers to get the comprehensive view needed to develop effective AI models. The very foundation of precision medicine—connected data—is missing.
This is the core challenge that federated learning solves. But to understand the solution, we must first grasp what keeps this data locked up.

The High Wall of Data Privacy and Regulation
Hospitals don’t share patient data because it’s incredibly risky and highly regulated. Your medical records contain deeply personal information, and protecting it is mandated by laws like HIPAA regulations in the U.S. and GDPR compliance in Europe.
These rules are critical, especially when 68% of healthcare organizations faced a data breach last year. For an institution, every data transfer introduces security risks and complex legal problems. Coordinating a data-sharing agreement between a London hospital and Boston researchers can take years, requiring legal teams to steer both GDPR and HIPAA. Faced with these obstacles, most institutions choose the safer option: keeping their data locked down. This prevents life-saving research from ever starting. Federated learning bypasses this by keeping data secure at its source, enabling collaboration without the risks of data transfer.
The Problem of Biased and Incomplete Data
Even when data is accessible, it’s often flawed. Many medical datasets lack diversity, underrepresenting women, ethnic minorities, and certain socioeconomic groups. AI models trained on this skewed data inherit these biases, leading to algorithms that fail for entire populations. This isn’t just poor science; it’s healthcare that amplifies existing inequities.
Data quality is another major issue. A staggering 80% of healthcare data is unstructured—buried in doctors’ notes and scanned images. Records are often incomplete, with inconsistent terminology across institutions. Training an AI on such messy data is like asking it to read a book with missing pages written in multiple languages. The resulting models are built on a flawed foundation, leading to inaccurate predictions and systematic failures for underserved groups.
Federated learning directly addresses this by enabling secure collaboration across many institutions. This creates virtual datasets that are larger, more diverse, and more representative, leading to fairer and more accurate AI without moving sensitive data.
Federated Learning: The Collaborative Bridge for Healthcare AI
Imagine hospitals across continents collaborating to cure rare diseases without a single patient record ever leaving its source. This is the reality of federated learning.
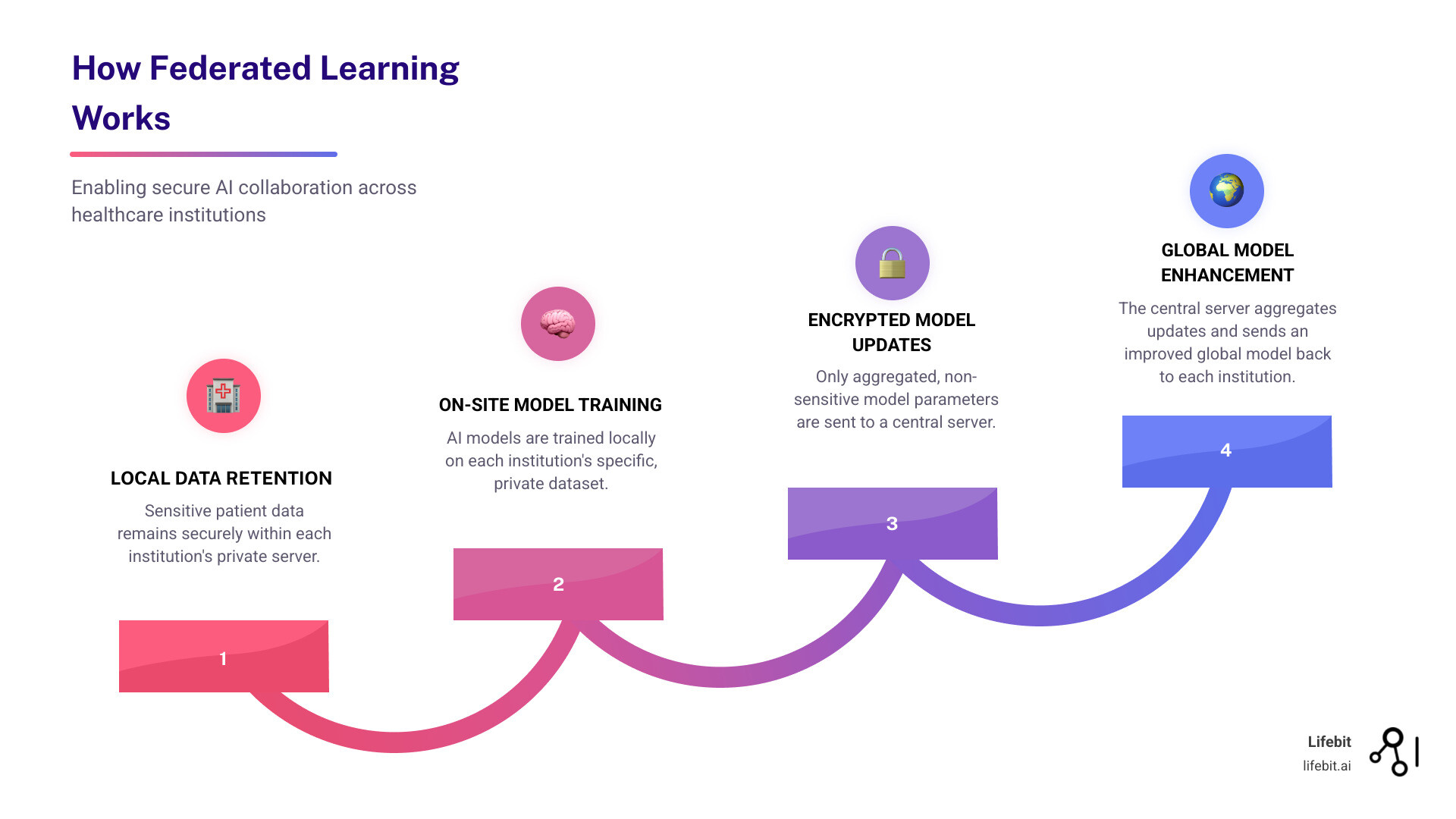
Federated learning is a privacy-preserving AI technique that reverses the traditional machine learning model. Instead of pooling data into a central, vulnerable database, it brings the AI model to where the data resides. The data stays locked down; only the insights travel.
The process is simple: each hospital trains an AI model on its local patient data. Instead of sharing the data, it shares only the encrypted model parameters—the lessons learned, not the patient stories. These updates are sent to a central server, which aggregates them into a smarter global model. This improved model is then sent back to each institution, and the cycle repeats.
The raw data never moves. This is the key. We gain the benefits of large-scale, diverse datasets without the privacy and security risks of traditional data sharing.
Enabling Secure Collaboration Across Institutions
Traditional multi-institutional research is a logistical nightmare of legal agreements and privacy problems. Federated learning changes this dynamic entirely. It allows institutions to contribute their knowledge while maintaining complete control over their patient data.
This shift enables a research team in Boston to collaborate with colleagues in London, Berlin, and Tel Aviv, creating a virtual dataset that spans continents. Each institution’s unique data strengthens the AI model, making it more robust and representative than any single organization could build alone. This also improves data equity, allowing smaller hospitals to participate in cutting-edge research. As research shows, federated learning is breaking down the barriers that have held back collaborative healthcare for decades by building the institutional trust needed for global health initiatives.
How Federated Learning Meets Precision Medicine: A Privacy-First Approach
At Lifebit, we believe privacy is the foundation of innovation. Federated learning embodies this by design. It maintains data sovereignty, meaning each institution retains absolute control over its data. The data never leaves their secure environment.
Privacy protections go even deeper. Advanced techniques like differential privacy add statistical noise to model updates, making it mathematically impossible to reverse-engineer individual patient information. Secure multi-party computation allows joint calculations on combined data while keeping each party’s input private. These overlapping shields create a robust defense against breaches.
This privacy-first approach is critical for precision medicine, which relies on highly sensitive genomic and clinical data. Federated learning provides a framework to extract insights from these datasets without compromising the trust that is sacred in healthcare. We can finally tap into the world’s collective medical knowledge to build more accurate and representative AI, all while keeping patient data more secure than ever.
Federated Learning Meets Precision Medicine: How Collaboration Is Powering Personalized Care
The convergence of federated learning and precision medicine is already delivering on its promise. By enabling secure, large-scale collaboration, we are making personalized medicine a reality for every patient.

Key Technical Considerations for Implementation
Implementing federated learning in healthcare requires addressing several technical challenges to ensure collaborative AI models deliver on their promise. Success depends on getting these factors right:
- Data Standardization: Data from different hospitals arrives in different formats. Harmonizing this data into a standard format (like the OMOP Common Data Model) is essential for models to make meaningful comparisons.
- EHR Interoperability: Electronic health record systems often don’t speak the same language. Addressing these compatibility gaps upfront is crucial for seamless data exchange, as noted in a systematic review of FL architectures.
- Data Quality Control: The principle of “garbage in, garbage out” is critical. Incomplete or inaccurate local datasets will degrade the global model. Ensuring data completeness and consistency is paramount.
- Communication Efficiency: While raw data stays put, model updates travel between sites. Slow networks can create bottlenecks, so efficient communication protocols are necessary, especially for complex models.
- Statistical Heterogeneity: Medical data is inherently diverse (non-IID). A model trained at a children’s hospital in Boston will see different data than one at a geriatric center in Tel Aviv. This requires advanced techniques to ensure the model performs well across all sites.
Building Fairer, More Representative AI Models
One of federated learning’s greatest strengths is its ability to reduce bias. Traditional AI models, often trained on homogenous datasets, can perpetuate health disparities. A model trained on data from white men may fail to diagnose heart disease in Black women.
Federated learning tackles this by training AI on vast, diverse, and geographically distributed datasets. By pooling insights from providers in London, Singapore, Canada, and the USA, we expose models to a wider spectrum of patient characteristics. This directly addresses data bias by incorporating data from underrepresented populations, leading to more equitable and accurate predictions for everyone. These models also show better model generalizability, performing more reliably across different clinical settings. By building fairer AI, we reduce health disparities and deliver on the true promise of precision medicine. Our AI for Data Harmonization initiatives at Lifebit directly address the data quality issues that cause bias.
Performance Showdown: Federated vs. Centralized Models
How do federated models perform against traditional centralized approaches? The evidence is compelling: they often match or exceed them, especially when local data is limited.
| Feature | Federated Learning | Centralized Learning |
|---|---|---|
| Data Privacy | High: Raw data never leaves local institution, only model updates are shared. | Low: Raw patient data must be aggregated in one location, increasing privacy risks. |
| Security | High: Distributed nature reduces single point of failure; advanced protocols (differential privacy, secure multi-party computation) improve security. | Moderate to Low: Centralized data repository is a prime target for cyberattacks. |
| Scalability | High: Easily scales to many participating institutions and large datasets without moving data. | Moderate: Requires massive infrastructure to store and process all data centrally; data transfer becomes a bottleneck. |
| Cost | Moderate: Distributed computation, but requires robust network infrastructure and specialized platforms. | High: Significant costs for data storage, transfer, and security of a central data lake. |
| Model Performance | Matches or Surpasses Centralized: Especially effective with limited local data or fragmented datasets; achieves 99% of centralized model quality. | High: Can achieve high performance if data is clean, diverse, and sufficient, but limited by data access challenges. |
| Implementation Complexity | Moderate to High: Requires coordination, standardization, and robust technical infrastructure across institutions. | Moderate: Simpler model training once data is centralized, but complex data acquisition and governance. |
| Data Access & Equity | High: Democratizes access to diverse data insights without transferring ownership, enabling equitable contributions from all institutions. | Low: Data ownership and access can be contentious, leading to data monopolies and limiting contributions from smaller institutions. |
| Latency (for real-time applications) | Lower: Model updates are smaller, faster to transmit; local processing can provide quicker insights for edge applications. | Higher: Large data transfers can introduce significant delays, especially for real-time analytics. |
| Resilience to Attacks | High: If one node is compromised, the overall model integrity is maintained; local data remains secure. | Low: A breach of the central repository can compromise all data and models. |
In one simulation, a federated framework achieved 92% accuracy compared to 89% for a centralized model, with lower latency (220 ms vs. 350 ms). It also proved more resilient to cyber threats, maintaining 85% accuracy under attack compared to just 62% for the centralized system. This combination of strong performance, privacy, and security makes federated learning a powerful tool for advancing precision medicine.
Real-World Impact: Use Cases and Future Frontiers
Federated learning is already delivering tangible breakthroughs in personalized care. By connecting researchers globally, it is making a measurable difference in how we diagnose and treat complex diseases.

Breakthroughs in Diagnosis and Treatment
Federated platforms are enabling researchers to tackle challenges previously blocked by data silos. The results speak for themselves:
-
Rare Disease Identification: By training models across multiple hospitals, federated learning can spot subtle diagnostic signals of rare diseases that are invisible within a single institution’s limited data. This is particularly crucial for conditions affecting 1 in 200,000 people, where no single center has enough patients to develop a robust diagnostic model.
-
Medical Imaging Analysis: In oncology, researchers are using federated learning to improve breast tumor classification, assess prostate cancer severity from MRIs, and pre-segment pulmonary nodules from CT scans. For instance, a federated network of 20 institutions successfully trained a model to detect brain tumors from MRI scans with an accuracy of 90.9%, outperforming models trained at any single institution. This collaboration allowed the model to learn from a diverse range of scanners and patient demographics, significantly improving its generalizability and reducing the risk of site-specific bias.
-
Brain Tumor Classification: Federated approaches are helping researchers better distinguish between tumor types and grades, leading to more precise and effective treatment planning. A notable project trained a model on data from 71 institutions across six continents to distinguish glioblastoma from lower-grade gliomas, achieving performance comparable to a model trained on centralized data without compromising privacy.
-
COVID-19 Response: During the pandemic, researchers rapidly developed AI models for COVID-19 diagnosis using chest radiographs by collaborating across institutions without centralizing patient data, proving the model’s value in public health crises.
-
Patient Journey Analysis: Federated platforms allow researchers to trace patient data—diagnostics, treatments, outcomes—across institutions to understand the full patient journey. By securely connecting longitudinal data from different care settings—from primary care clinics to specialized cancer centers—researchers can build a holistic view of disease progression and treatment efficacy. This enables the identification of critical intervention points and the development of care pathways that are optimized for better patient outcomes, something impossible when data remains fragmented.
-
Predictive Modeling: By training on distributed EHR data, researchers can predict patient deterioration, optimize clinical trial site selection, and forecast tumor recurrence, enabling proactive interventions. For example, a federated model can predict the likelihood of sepsis in ICU patients 12 hours in advance by learning from real-time data across a hospital network, allowing for early and life-saving treatment.
The Future of Federated Learning in Precision Medicine
The future of federated learning is bright, with several exciting trends ready to further transform personalized healthcare.
-
Integration with IoT and Wearables: Imagine AI models learning from your smartwatch and home monitoring devices without your health data ever leaving your control. This enables highly personalized, proactive health insights, such as predicting a hypoglycemic event in a diabetic patient based on continuous glucose monitoring data federated across thousands of users.
-
Real-Time Clinical Trial Matching: Federated learning can identify eligible patients for clinical trials across a network of hospitals in real-time, dramatically accelerating recruitment and bringing new therapies to market faster. This also democratizes trial access, allowing patients in smaller or remote clinics to be matched with cutting-edge studies they would otherwise miss.
-
Personalized Drug Findy: By analyzing treatment responses across vast, diverse patient populations, federated learning can help identify novel drug targets and predict individual drug efficacy, speeding up the development of more effective medicines. This approach can uncover why a drug is effective in one sub-population but not another, paving the way for truly personalized pharmacology.
-
Predictive Public Health Surveillance: Health authorities can use federated models to track and predict disease outbreaks in real-time across cities and countries, enabling rapid, evidence-based interventions without compromising individual privacy. This could be used to monitor the spread of influenza strains or antimicrobial resistance patterns, providing a global view while keeping all data local.
A key enabler for this future is blockchain technology. By providing an immutable, auditable record of model updates and data access, blockchain-enabled federated learning improves security, transparency, and trust. This creates immutable audit trails that show exactly who accessed what and when, addressing critical privacy concerns and building confidence through transparency. This is especially important for establishing accountability in multi-stakeholder collaborations involving pharmaceutical companies, hospitals, and regulatory bodies.
Navigating the Challenges of Implementation
Federated learning is powerful, but implementation requires careful planning. Getting a global network of hospitals to work in harmony presents real challenges. The good news is that these challenges are well-understood and entirely surmountable with the right strategy.
Common Pitfalls and How to Avoid Them
Deploying federated learning can be complex. A comprehensive survey highlights common obstacles and, more importantly, how to overcome them.
-
Challenge: Statistical Heterogeneity. Medical data is notoriously non-IID (non-identically distributed). Data from a children’s hospital in London looks different from a geriatric center in Singapore due to differences in patient demographics, clinical practices, and imaging equipment. Solution: Advanced Data Harmonization and Algorithmic Adaptation. Before training, data must be transformed into a standardized format. This involves more than just mapping terminologies; it requires sophisticated ETL (Extract, Transform, Load) pipelines that can process and standardize diverse data types, from genomic sequences to clinical notes, into a common data model like OMOP. This foundational step ensures that the global model is learning from consistent, comparable information. Additionally, advanced federated algorithms like FedProx or SCAFFOLD can be used to correct for this heterogeneity during training, ensuring the final model performs well across all participating sites.
-
Challenge: Communication Costs. Sending model updates between hundreds of institutions can create significant network traffic and slow down the process, especially for hospitals with limited bandwidth. Solution: Asynchronous Learning and Model Compression. Instead of requiring all participants to update the model simultaneously (synchronous learning), asynchronous protocols allow each institution to contribute updates at its own pace. This not only improves efficiency and reduces bottlenecks but also increases inclusivity. Furthermore, techniques like model quantization and sparsification can be used to compress the size of the model updates, reducing the amount of data that needs to be transmitted without significantly impacting performance.
-
Challenge: Model Poisoning Attacks. A malicious actor could send corrupted model updates to degrade the global model’s performance or introduce biases. Solution: Robust Security, Governance, and Anomaly Detection. A strong Federated Data Governance framework is the first line of defense. This includes vetting participants and establishing clear rules for engagement. Technically, the central server can use anomaly detection algorithms to inspect incoming model updates, flagging or rejecting those that deviate significantly from the norm. Combining this with differential privacy, which adds noise to the updates, can also make it more difficult for an attacker to influence the final model in a targeted way.
-
Challenge: Lack of Standardization. Hospitals use different EHR systems, coding standards (e.g., ICD-9 vs. ICD-10), and data structures, making collaboration difficult. Solution: A Centralized Governance and Harmonization Strategy. Establishing clear rules of the road and using a platform that enforces data standards across all participants is key. This requires a dedicated effort to create and maintain a common data dictionary and transformation scripts that can be deployed at each local site. This upfront investment in standardization is critical for ensuring the quality and integrity of the federated learning process and the resulting AI model.
-
Challenge: System Complexity. Setting up a federated network requires sophisticated infrastructure, security, and coordination, which can be overwhelming for institutions with limited IT resources. Solution: Choosing the Right Platform and Partner. This is not a DIY project. A managed platform like Lifebit’s Federated Trusted Research Environment handles the technical complexity, security, and compliance. By providing pre-configured environments, standardized workflows, and dedicated support, such platforms abstract away the infrastructural hurdles, allowing researchers to focus on science, not system administration.
Conclusion: A New Era of Personalized, Collaborative Healthcare
Federated learning is fundamentally changing healthcare. The data walls that once stalled research are coming down, and fragmented datasets are being connected—all without compromising patient confidentiality. This is the power of secure collaboration.
When a hospital in New York can contribute insights to the same AI model as a research center in London and a clinic in Singapore, we create AI that understands the full spectrum of human diversity. We build treatments that work for everyone.
This shift democratizes data access, enables research on rare diseases, and accelerates drug findy. It’s happening now, and it’s built on the principle that we can share knowledge without sharing data.
At Lifebit, we are at the forefront of this change. Our next-generation federated AI platform enables pharmaceutical companies, government agencies, and research institutions to collaborate at a global scale. Through our Trusted Research Environment (TRE), organizations conduct secure research across distributed datasets. Our Trusted Data Lakehouse (TDL) harmonizes disparate data into analysis-ready formats, and our R.E.A.L. (Real-time Evidence & Analytics Layer) delivers AI-driven insights across hybrid data ecosystems.
This represents a fundamental shift from isolated institutions to collaborative networks, and from one-size-fits-all treatments to truly personalized medicine. The future of healthcare is collaborative, personalized, and federated.
The question isn’t whether federated learning will change precision medicine. It already has. The question is: will you be part of this change?
Learn more about our Trusted Research Environment solutions and find out how your organization can join this new era of collaborative healthcare.

