Beginner’s Guide to OMOP

Why OMOP is Changing Healthcare Data Analysis
OMOP (Observational Medical Outcomes Partnership) is a standardized data model that transforms disparate healthcare databases into a unified format, enabling researchers to conduct large-scale, reproducible studies across multiple data sources. Here’s what you need to know:
Quick Facts:
- What it is: A common data model that standardizes healthcare data structure and content
- Purpose: Enables systematic analysis of observational databases from different sources
- Key benefit: Allows the same analysis code to run across multiple datasets
- Current version: CDM v5.4, maintained by the OHDSI community
- Global reach: Adopted by major healthcare systems including the All of Us Research Program and over 150 Veterans Health Administration medical centers
Healthcare data has historically been fragmented across organizations, with each system using different formats, terminologies, and structures. When every healthcare system uses a different name for a data field – like “systolic blood pressure” or “blood glucose level” – comparing and analyzing data across systems becomes nearly impossible.
The OMOP Common Data Model solves this problem by creating a standardized structure that accommodates both administrative claims data and electronic health records. This standardization enables researchers to generate evidence from diverse observational data sources while maintaining minimal information loss during the change process.
The model can scale to handle databases with hundreds of millions of patients and billions of clinical observations, making it ideal for large-scale pharmacovigilance, comparative effectiveness research, and real-world evidence generation.
As Maria Chatzou Dunford, CEO and Co-founder of Lifebit, I’ve seen how standardized data models like OMOP enable breakthrough findies in precision medicine and drug development. My experience building federated data platforms has shown me that OMOP’s approach to data harmonization is essential for open uping the full potential of global healthcare data.
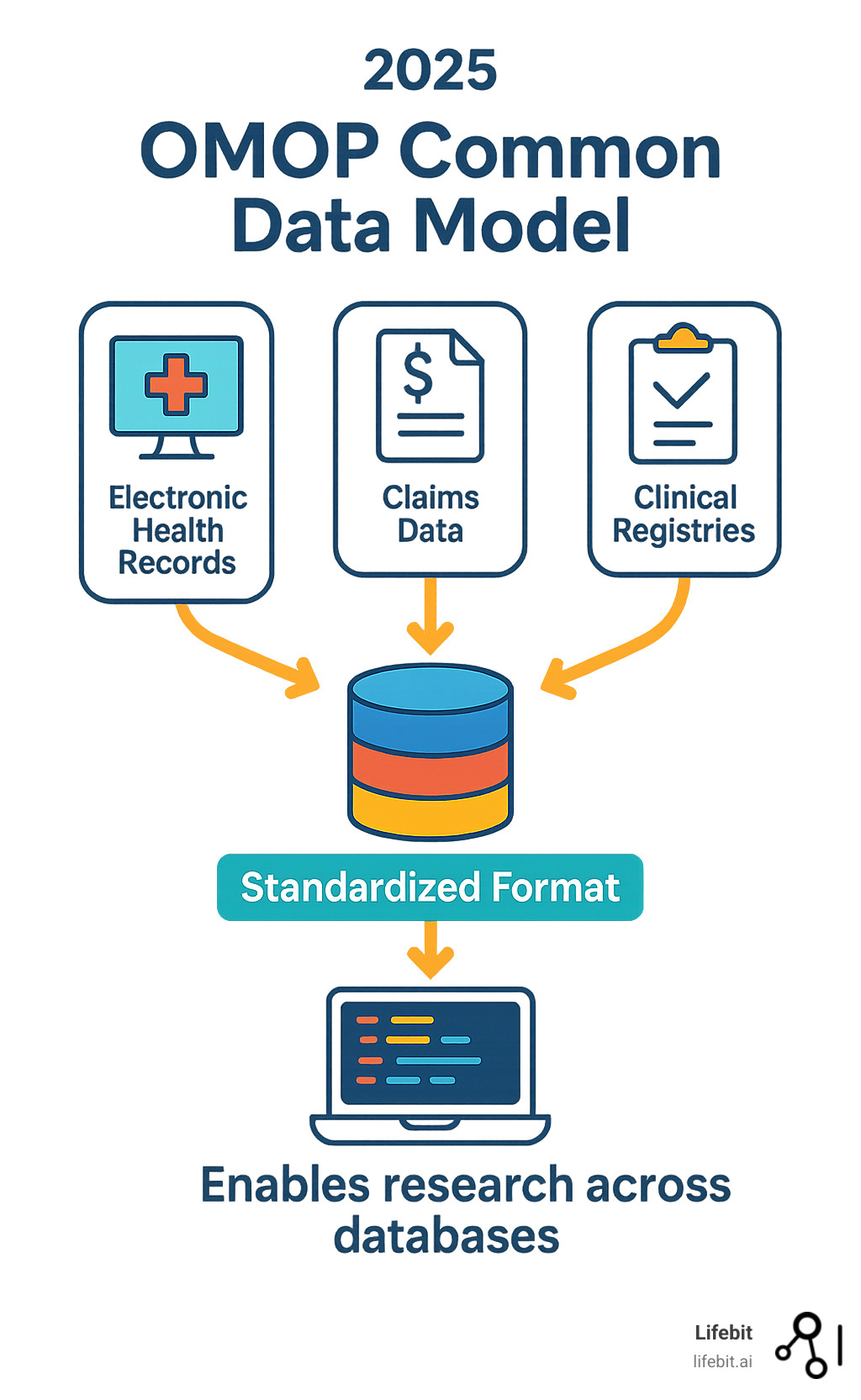
What is the OMOP Common Data Model (CDM)?
Imagine trying to have a conversation where everyone speaks a different language. That’s essentially what healthcare data looked like before the OMOP Common Data Model came along. Every hospital, insurance company, and research center had their own way of storing and organizing patient information, making it nearly impossible to learn from data across different systems.
The Observational Medical Outcomes Partnership (OMOP) started as an ambitious 5-year project bringing together an unlikely alliance: the FDA, pharmaceutical companies, and healthcare providers. Their shared frustration? The inability to properly study how medical treatments actually work in the real world because everyone’s data was locked in incompatible formats.
This public-private partnership had a clear mission: figure out how to use observational healthcare databases to study the effects of medical products. Think of it as trying to solve a massive puzzle where all the pieces came from different boxes with different rules.
The project was remarkably successful. OMOP proved that you could create a common infrastructure capable of handling observational data from completely different sources around the world. After completing its initial goals in 2013, the project transitioned to the Reagan-Udall Foundation, but its most important legacy – the Common Data Model – found a new home with the OHDSI community.
The OMOP CDM works like a universal translator for healthcare data. It takes information stored in wildly different formats and transforms it into a common language that researchers can understand and analyze systematically. This standardization covers both the structure (how data is organized) and content (what the data actually means), enabling efficient analyses that produce reliable evidence.
For healthcare organizations wrestling with data integration challenges, understanding Health Data Interoperability and Data Harmonization: Overcoming Challenges provides essential context for why this standardization matters so much.
The Challenge: Why a Common Data Model is Necessary
Healthcare organizations have been building databases for decades, but they’ve been doing it in isolation. Each system developed its own approach, creating a landscape of data silos that make large-scale research incredibly difficult.
The problem runs deeper than just different software systems. Consider how Electronic Medical Records (EMR) work compared to claims data. EMRs are designed to help doctors and nurses provide patient care. They capture detailed clinical information organized around the workflow needs of healthcare providers – what symptoms did the patient report, what tests were ordered, how did they respond to treatment.
Administrative claims data serves a completely different purpose. It’s built for insurance reimbursement, focusing on what procedures were performed and what diagnoses justify payment. While claims data covers large populations, it often lacks the clinical detail that EMRs provide.
These fundamental differences create serious barriers to research. Varying formats mean that a blood pressure reading might be stored completely differently across systems. Different terminologies mean the same medical condition could be coded using entirely different systems. Inconsistent structures make it nearly impossible to combine data from multiple sources.
The result? Inefficient research where teams spend months just preparing data before they can begin their actual analysis. Studies lack reproducibility because findings from one database can’t be easily verified using data from another institution. Large-scale analytics become virtually impossible when every dataset requires custom handling.
The Solution: How the OMOP CDM Works
The OMOP CDM takes a brilliant approach to this mess: instead of forcing healthcare systems to completely overhaul their operational databases, it creates a separate, standardized analytical layer.
Here’s the key insight – the CDM focuses on changing data rather than changing systems. Healthcare organizations keep their existing workflows, but they transform a copy of their data into the OMOP format for research purposes.
This change involves creating a standardized structure where all data is organized into predefined tables with consistent relationships. Medical concepts get mapped to standardized vocabularies, so a heart attack is coded the same way whether it comes from a hospital in Boston or a clinic in Berlin.
The real magic happens with standardized content. The same analysis code can run across multiple datasets without modification. This enables what researchers call “federated analysis” – you can conduct identical studies across multiple institutions without anyone having to share their raw data.
The approach preserves minimal information loss during the change process. Original codes are maintained alongside standardized ones, so researchers can always trace back to the source data if needed.
This system enables reliable evidence generation at scale. Instead of spending months customizing analysis code for each new dataset, researchers can write their analysis once and run it everywhere. It’s like having one analysis code for multiple datasets, dramatically speeding up the research process.
For organizations looking to implement this approach, our guide on Creating Research-Ready Health Data provides practical insights for getting started.
How the OMOP CDM Standardizes Healthcare Data
Think of the OMOP CDM as a master blueprint that tells every healthcare database how to organize its information in exactly the same way. It’s like having a universal filing system that works whether you’re dealing with a small clinic’s patient records or a massive hospital network’s data warehouse.
The model follows a person-centric approach, which means everything revolves around individual patients and their healthcare journeys over time. This design lets researchers track how someone’s health evolves – from their first diagnosis through treatments, complications, and outcomes – creating a complete picture of their medical story.
The current version, CDM v5.4, took a full year to develop with input from healthcare experts around the world. It includes 39 standardized tables that work together like pieces of a puzzle, each capturing different aspects of healthcare data while maintaining perfect compatibility with each other.
What makes this approach so powerful is how it handles the change process. When hospitals and health systems convert their data to the OMOP format, they don’t lose any important information. Instead, they gain the ability to use the same analytical tools and methods that work across every other OMOP database in the world.
For the complete technical details, you can Read more about the OMOP Common Data Model.
The Core Components of the OMOP CDM
The OMOP CDM organizes healthcare information into logical groups that mirror how we think about patient care. At the center of everything is the Person table, which contains basic demographic information like age, gender, and race for each individual in the database.
The Visit Occurrence table captures every time someone interacts with the healthcare system – whether it’s a routine checkup, an emergency room visit, or a week-long hospital stay. This table creates the timeline that connects all other healthcare events.
Clinical events get their own dedicated spaces: the Condition Occurrence table records every diagnosis and medical condition, while the Drug Exposure table tracks all medications prescribed or administered. The Procedure Occurrence table documents medical interventions, and the Measurement table stores laboratory results, vital signs, and other quantitative health data.
For everything else that doesn’t fit neatly into these categories, the Observation table serves as a catch-all for additional clinical facts and notes. This flexibility ensures that no important healthcare information gets lost in the change process.
The model also includes health system data tables that provide crucial context. These tables tell you where care was delivered, which providers were involved, and how different healthcare locations connect to each other.
Perhaps most cleverly, the OMOP CDM includes derived elements that make analysis easier. These include Drug Era, Condition Era, and Dose Era tables that group related events into meaningful time periods. Instead of looking at individual prescription fills, researchers can analyze entire treatment periods.
The Role of Standardized Vocabularies in OMOP
Here’s where OMOP gets really smart about solving the “Tower of Babel” problem in healthcare data. Every medical concept – from “chest pain” to “acetaminophen 500mg” – gets translated into a universal language that every OMOP database can understand.
The system uses established medical vocabularies like SNOMED-CT for clinical conditions, RxNorm for medications, and LOINC for laboratory tests. But it doesn’t stop there. The OMOP vocabulary system creates a massive crosswalk that connects all these different coding systems together.
Imagine two hospitals: one codes diabetes using ICD-10 codes, while another uses ICD-9 codes from their older system. In the OMOP world, both of these codes get mapped to the same standard SNOMED concept for diabetes. This means researchers can find all diabetes patients across both hospitals without having to know anything about the different coding systems each hospital uses.
The ATHENA vocabulary browser serves as the central hub for all these mappings. You can Explore the OHDSI vocabularies on ATHENA to see how medical concepts from different systems connect to each other. It’s like having a universal translator for medical terminology.
This standardization creates something remarkable: analytical code that works everywhere. Write a study looking for patients with heart failure, and that same code will run on any OMOP database, whether it’s from a hospital in New York or a clinic in London.
The vocabulary system also preserves the original source codes alongside the standardized ones. This means organizations don’t lose their native coding information when they adopt OMOP – they just gain the ability to speak the same language as everyone else.
For organizations tackling the technical side of this change, our guide on Health Data Standardisation: Technical Challenges walks through the practical steps involved in implementing these vocabulary mappings.
The Evolution and Governance of the OMOP Standard
The original OMOP project launched with ambitious goals that seemed almost impossible at the time. How do you take healthcare data from completely different systems and make them work together? The five-year initiative brought together an unlikely alliance of the FDA, pharmaceutical companies, and healthcare providers – groups that don’t always see eye to eye.
Their mission was straightforward but challenging: inform the appropriate use of observational healthcare databases for studying the effects of medical products. What they created was something much bigger – a foundation that would transform how we think about healthcare data research.
The project succeeded beyond expectations, proving that you could establish a common research infrastructure capable of handling observational data from anywhere in the world. When the original OMOP project wrapped up, it transitioned to the Reagan-Udall Foundation in 2013, but its most important legacy was just getting started.
The OMOP project had three core aims that continue to guide development today. First, they wanted to conduct methodological research – essentially figuring out which analytical methods actually work for identifying real medical associations while avoiding false alarms. Second, they focused on tool development, creating practical capabilities for changing, understanding, and analyzing different data sources. Finally, they envisioned a shared resource that would bring the entire research community together.
You can learn more about this fascinating origin story at Welcome to OMOP.
From OMOP Project to the OHDSI Community
When the original OMOP project ended, something remarkable happened. Instead of fading away, it evolved into something even more powerful – the Observational Health Data Sciences and Informatics (OHDSI) collaborative.
OHDSI kept all the original OMOP research investigators but opened the doors to a much broader community. Today, it’s a global network of researchers, clinicians, and data scientists who share a common vision: making observational research accessible to everyone.
The community has grown organically, with members who develop and maintain the OMOP CDM, create analytical tools and methods, share research findings and best practices, and provide support and training to newcomers. It’s like having a worldwide team of experts who are genuinely excited to help you succeed.
Every year, the OHDSI symposium brings together hundreds of participants from around the world. These aren’t just dry academic conferences – they’re energetic gatherings where people share breakthrough research, debate methodological advances, and plan the future of observational health research. The collaborative spirit is infectious, and it’s what makes OMOP more than just a technical standard.
If you’re curious about joining this vibrant community, Learn more about the OHDSI community.
How the CDM is Maintained and Updated
The OMOP CDM isn’t a static document gathering dust on a shelf. It’s a living model that evolves based on real-world needs and community feedback. The OHDSI CDM Working Group keeps everything running smoothly, responding to requests from researchers who are actually using the model in their daily work.
The update process is refreshingly transparent and democratic. Community members propose changes through GitHub issues, just like any modern software project. The CDM Working Group evaluates these proposals, considering their impact and feasibility. Proposed changes are shared with the broader community for feedback – no ivory tower decisions here. Finally, approved changes are implemented and delivered through an R package that makes updates easy to deploy.
Currently, the CDM Working Group maintains over 3,500 data quality checks against OMOP CDM instances. That’s a lot of quality control, but it ensures that when you’re working with OMOP data, you can trust its integrity.
The current version is CDM v5.4, which was developed over an entire year with input from the global community. Eight OHDSI tools support this version, with different levels of support that reflect a thoughtful approach to compatibility.
One of the smartest decisions the Working Group made was their commitment to backwards compatibility. This means you can create older CDM versions from newer ones without losing any information. It protects existing investments while enabling continuous improvement – exactly what you want in a standard that organizations depend on.
Want to help shape the future of OMOP? You can Join the CDM Working Group and contribute to the model’s ongoing development.
Benefits and Real-World Applications

The OMOP CDM has revolutionized how healthcare organizations approach data analysis, turning what was once a months-long data preparation nightmare into a streamlined research process. The impact goes far beyond simple convenience – it’s fundamentally changing how we generate evidence from real-world healthcare data.
When researchers can focus on the science instead of wrestling with data formats, breakthrough findies happen faster. The reproducibility that OMOP enables means that studies can be validated across multiple institutions, creating more robust evidence for clinical decision-making.
The transparency built into the model ensures that analytical methods can be shared and scrutinized by the global research community. This openness accelerates scientific progress and helps identify potential issues before they affect patient care.
Perhaps most importantly, OMOP enables collaboration at a scale previously impossible. Researchers can now conduct studies across continents without the traditional barriers of data incompatibility. The model’s scalability means it can handle databases with hundreds of millions of patients and billions of clinical observations – making it perfect for population-level research.
This combination of features enables large-scale network studies for pharmacovigilance and comparative effectiveness research that would be impossible with traditional approaches. For a deeper dive into these advantages, explore our analysis of the Seven Benefits of Health Data Standardisation.
Key Benefits for Researchers and Organisations
The change that OMOP brings to research workflows is remarkable. Faster study setup becomes possible because data is already in a research-ready format. Instead of spending months harmonizing different data sources, researchers can begin analysis immediately after gaining access to an OMOP database.
The re-use of analytical tools represents another game-changing advantage. The OHDSI community has developed a comprehensive library of analytical tools that work seamlessly across all OMOP databases. These tools support sophisticated analyses including patient-level prediction for building models that forecast individual patient outcomes, population-level effect estimation for assessing treatment effects across large populations, and phenotyping for defining and identifying patient cohorts based on complex clinical characteristics.
Access to a global data network creates natural opportunities for collaboration that didn’t exist before. When multiple institutions use the same data model, researchers can run identical studies across different sites, dramatically increasing sample sizes and improving the generalizability of findings.
The standardization process itself often leads to improved data quality. The OHDSI Data Quality Dashboard runs over 3,500 checks to ensure data integrity, catching issues that might otherwise go unnoticed. This quality assurance process means researchers can trust their results and stakeholders can have confidence in the evidence generated.
OMOP also democratizes access to complex healthcare data analysis. By standardizing complex phenotypic data into a common format, the model makes analysis-ready datasets accessible to researchers who might not have the technical expertise to handle raw healthcare data from multiple sources.
OMOP in Action: Global Adoption and Impact
The real-world impact of OMOP becomes clear when you see how major healthcare systems and research initiatives have acceptd the standard. These implementations demonstrate that OMOP isn’t just a theoretical framework – it’s a practical solution that works at massive scale.
The All of Us Research Program represents one of the most ambitious implementations of OMOP. This initiative uses OMOP Common Data Model Version 5 infrastructure to standardize data from over one million participants. The program demonstrates OMOP‘s versatility by handling diverse data types including physical measurements, electronic health records, and participant-provided information – all unified under a single analytical framework.
The Veterans Health Administration has transformed data from over 150 medical centers into the OMOP format, creating one of the world’s largest standardized healthcare databases. This implementation enables researchers to study veteran health outcomes at unprecedented scale, supporting everything from mental health research to chronic disease management studies.
University of California medical centers have acceptd OMOP across their system, with UC Davis, UC Irvine, UC Los Angeles, UC San Diego, and UC San Francisco all implementing the standard. This creates a powerful research network across California’s academic medical centers, enabling collaborative studies that span diverse patient populations.
The momentum is building internationally as well. The NHS Research Secure Data Environment Network in the UK has agreed to adopt OMOP as a common data model, while the EHDEN consortium has supported the change of numerous European databases to OMOP format.
This global adoption creates a powerful network effect – as more institutions implement OMOP, the value of the standard increases exponentially for everyone involved. Researchers gain access to larger, more diverse datasets, while healthcare systems benefit from shared analytical tools and methodologies. You can read more about this growing momentum in the announcement that the NHS Research SDE network agrees to adopt common data model.
Getting Started: Tools and Resources
Ready to dive into OMOP data? The good news is that the OHDSI community has built an incredible ecosystem of open-source tools that make working with standardized healthcare data surprisingly accessible – even if you’re just starting out.
Think of it like learning to cook in a well-equipped kitchen. All the tools are there, the recipes are proven, and there’s a friendly community of chefs ready to help when you get stuck. Whether you’re a seasoned data scientist or a clinician taking your first steps into healthcare analytics, there’s a clear path forward.
The collaborative spirit of OHDSI means you’re never working alone. When you hit a roadblock (and everyone does), there’s likely someone in the community who’s faced the same challenge and figured out a solution. This shared knowledge makes the learning curve much gentler than you might expect.
For organizations ready to implement OMOP, The Book of OHDSI is your comprehensive guide. It covers everything from basic concepts to advanced analytical methods, written in a way that actually makes sense.
If you’re dealing with broader clinical data integration challenges, our guide on Clinical Data Integration Software provides additional context that complements your OMOP journey.
Essential OHDSI Tools for Working with OMOP
The OHDSI community has crafted a suite of tools that work together like a well-orchestrated symphony. Each tool has its role, but they’re designed to complement each other seamlessly.
ATLAS is your command center – a web-based interface that lets you define patient populations and design studies without writing a single line of code. It’s surprisingly intuitive once you get the hang of it, making complex cohort definitions feel like filling out a form.
Achilles acts as your data detective, generating comprehensive reports about what’s actually in your OMOP database. Think of it as getting a detailed map before you start exploring new territory – it shows you what treasures are available and where potential pitfalls might be hiding.
The Data Quality Dashboard is your quality control expert, running over 3,500 automated checks to ensure your data meets research standards. It’s like having a meticulous editor review your work, catching issues before they become problems.
White Rabbit & Rabbit-In-A-Hat support the change process with a touch of whimsy in their naming. These tools help data engineers understand source data structures and design the conversion to OMOP format. The rabbit theme makes the often-tedious ETL process a bit more fun.
FeatureExtraction empowers you to build the building blocks for predictive modeling and population studies. It’s the tool that turns raw OMOP data into the features that fuel advanced analytics.
Cohort Diagnostics helps you validate your patient definitions and assess how they perform across different databases. It’s like having a quality assurance team that ensures your research definitions work consistently everywhere.
These tools create a complete workflow from data preparation through analysis and reporting. You can Explore all OHDSI software tools to find the perfect combination for your specific research needs.
How to Join and Contribute to the Community
Joining the OHDSI community feels less like entering an exclusive club and more like being welcomed into a global family of researchers who share your passion for improving healthcare through data.
The OHDSI Forums are your starting point for getting help and sharing experiences. They’re organized by topic, so you can easily find discussions relevant to your specific challenges. Don’t be shy about asking questions – the community genuinely enjoys helping newcomers find their footing.
Working Groups focus on specific aspects of the OMOP ecosystem, from CDM development to analytical methods. These groups meet regularly and always welcome fresh perspectives. Whether you’re interested in technical development or methodological research, there’s likely a group that matches your interests.
Community Calls offer regular opportunities to learn about new developments and connect with other researchers. These calls often feature presentations of cutting-edge research using OMOP data, giving you inspiration for your own projects.
Contributing to Development can take many forms – proposing changes to the CDM, developing new analytical tools, or improving documentation. Every contribution, no matter how small, helps strengthen the entire ecosystem.
Attending Symposia provides invaluable opportunities for in-person networking and learning about the latest research. These events showcase the incredible diversity of work being done with OMOP data worldwide.
Sharing Research helps build the collective knowledge base and demonstrates the real-world impact of standardized observational research. When you publish studies using OMOP data, you’re contributing to a growing body of evidence about the model’s effectiveness.
The community operates on principles of openness, collaboration, and scientific rigor. There’s no hierarchy based on institutional affiliation or years of experience – good ideas and helpful contributions are valued regardless of their source.
Ready to take your first step? Post questions on the OHDSI Forum where community members are genuinely excited to help newcomers steer OMOP and observational research. You’ll be surprised how quickly you go from asking questions to helping others find their way.
Conclusion
The OMOP Common Data Model has fundamentally changed how we think about healthcare data analysis. What started as a solution to fragmented, incompatible databases has become something much more powerful – a global movement toward collaborative, reproducible research.
The real magic of OMOP isn’t just in its technical design, though that’s certainly impressive. It’s in how it has brought together researchers, clinicians, and data scientists from around the world who share a common vision. Through the OHDSI community, what could have been just another data standard has become a thriving ecosystem of tools, methods, and shared knowledge.
Looking ahead, the need for standardized data models like OMOP will only grow stronger. Healthcare data is exploding – from electronic health records to genomic sequences to real-world evidence from wearable devices. Without sophisticated approaches to harmonization and analysis, we risk drowning in data while thirsting for insights.
At Lifebit, we’ve witnessed how OMOP standardization opens up breakthrough findies in precision medicine and drug safety. Our federated AI platform builds on the foundation that OMOP provides, enabling secure, real-time analysis across global biomedical datasets. When you combine OMOP‘s standardization with advanced AI/ML analytics and federated governance, you can uncover insights that would be impossible with traditional approaches.
The future of observational research depends on our ability to collaborate across institutional and national boundaries. OMOP provides the technical foundation for this collaboration, but it’s the community of dedicated researchers who make it truly transformative.
Whether you’re a researcher eager to expand your analytical capabilities, a healthcare organization wanting to participate in multi-site studies, or a technology company building next-generation health analytics tools, OMOP offers a proven pathway forward. The journey from fragmented healthcare data to unified, analysis-ready datasets isn’t always easy, but the rewards are enormous – better science, improved patient outcomes, and more efficient healthcare systems.
OMOP has shown us what becomes possible when we work together toward common goals. It’s proof that standardization doesn’t have to mean limitation – it can mean liberation from the technical barriers that have held back medical research for too long.
Ready to explore how standardized healthcare data can transform your research? Explore Lifebit’s federated platform for real-world data analysis and find how we’re helping organizations worldwide harness the power of OMOP and other standardized data models for breakthrough findies in precision medicine and beyond.

